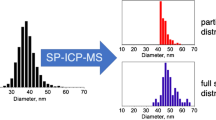
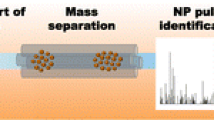
Abstract
Biological interactions, toxicity, and environmental fate of engineered nanoparticles are affected by colloidal stability and aggregation. To assess nanoparticle aggregation, analytical methods are needed that allow quantification of individual nanoparticle aggregates. However, most techniques used for nanoparticle aggregation analysis are limited to ensemble measurements or require harsh sample preparation that may introduce artifacts. An ideal method would analyze aggregate size in situ with single-nanoparticle resolution. Here, we established and validated single-particle inductively coupled plasma mass spectrometry (SP-ICP-MS) as an unbiased high-throughput analytical technique to quantify nanoparticle size distributions and aggregation in situ. We induced nanoparticle aggregation by exposure to physiologically relevant saline conditions and applied SP-ICP-MS to quantify aggregate size and aggregation kinetics at the individual aggregate level. In situ SP-ICP-MS analysis revealed rational surface engineering principles for the preparation of colloidally stable nanoparticles. Our quantitative SP-ICP-MS technique is a platform technology to evaluate aggregation characteristics of various types of surface-engineered nanoparticles under physiologically relevant conditions. Potential widespread applications of this method may include the study of nanoparticle aggregation in environmental samples and the preparation of colloidally stable nanoparticle formulations for bioanalytical assays and nanomedicine.

Graphical abstract





Similar content being viewed by others
References
Pelaz B, Alexiou C, Alvarez-Puebla RA, et al. Diverse applications of nanomedicine. ACS Nano. 2017;11:2313–81. https://doi.org/10.1021/acsnano.6b06040.
Wilhelm S. Perspectives for upconverting nanoparticles. ACS Nano. 2017;11:10644–53.
Narum SM, Le T, Le DP, et al. Passive targeting in nanomedicine: fundamental concepts, body interactions, and clinical potential. In: Nanoparticles for Biomedical Applications: Elsevier; 2020. p. 37–53.
Albanese A, Walkey CD, Olsen JB, et al. Secreted biomolecules alter the biological identity and cellular interactions of nanoparticles. ACS Nano. 2014;8:5515–26. https://doi.org/10.1021/nn4061012.
Wilhelm S, Tavares AJ, Dai Q, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:1–12.
Poon W, Zhang YN, Ouyang B, et al. Elimination pathways of nanoparticles. ACS Nano. 2019;13:5785–98. https://doi.org/10.1021/acsnano.9b01383.
Donahue ND, Acar H, Wilhelm S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv Drug Deliv Rev. 2019;143. https://doi.org/10.1016/j.addr.2019.04.008.
Modena MM, Rühle B, Burg TP, Wuttke S. Nanoparticle characterization: what to measure? Adv Mater. 2019;31:1901556. https://doi.org/10.1002/adma.201901556.
Marquis BJ, Love SA, Braun KL, Haynes CL. Analytical methods to assess nanoparticle toxicity. Analyst. 2009;134:425–39.
Hoo CM, Starostin N, West P, Mecartney ML. A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J Nanopart Res. 2008;10:89–96. https://doi.org/10.1007/s11051-008-9435-7.
Olson J, Dominguez-Medina S, Hoggard A, et al. Optical characterization of single plasmonic nanoparticles. Chem Soc Rev. 2015;44:40–57.
Montaño MD, Lowry GV, Blue J. Current status and future direction for examining engineered nanoparticles in natural systems. 2010. https://doi.org/10.1071/EN14037.
Brar SK, Verma M. Measurement of nanoparticles by light-scattering techniques. TrAC - Trends Anal Chem. 2011;30:4–17.
Dastanpour R, Boone JM, Rogak SN. Automated primary particle sizing of nanoparticle aggregates by TEM image analysis. Powder Technol. 2016;295:218–24. https://doi.org/10.1016/j.powtec.2016.03.027.
Filipe V, Hawe A, Jiskoot W. Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm Res. 2010;27:796–810. https://doi.org/10.1007/s11095-010-0073-2.
Montaño MD, Olesik JW, Barber AG, et al. Single particle ICP-MS: advances toward routine analysis of nanomaterials. Anal Bioanal Chem. 2016;408:5053–74. https://doi.org/10.1007/s00216-016-9676-8.
Mozhayeva D, Engelhard C. A critical review of single particle inductively coupled plasma mass spectrometry – a step towards an ideal method for nanomaterial characterization. J Anal At Spectrom. 2020. https://doi.org/10.1039/c9ja00206e.
Corte Rodríguez M, Álvarez-Fernández García R, Blanco E, et al. Quantitative evaluation of cisplatin uptake in sensitive and resistant individual cells by single-cell ICP-MS (SC-ICP-MS). Anal Chem. 2017;89:11491–7. https://doi.org/10.1021/acs.analchem.7b02746.
Wilhelm S, Bensen RC, Kothapali NR, et al (2018) Quantification of gold nanoparticle uptake into cancer cells using single cell ICP-MS. PerkinElmer Appl Note.
Lee JC, Donahue ND, Mao AS, et al. Exploring maleimide-based nanoparticle surface engineering to control cellular interactions. ACS Appl Nano Mater. 2020;3:2421–9. https://doi.org/10.1021/acsanm.9b02541.
Albanese A, Tang PS, Chan WCW. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. https://doi.org/10.1146/annurev-bioeng-071811-150124.
Wilhelm S, Kaiser M, Würth C, et al. Water dispersible upconverting nanoparticles: effects of surface modification on their luminescence and colloidal stability. Nanoscale. 2015;7:1403–10. https://doi.org/10.1039/c4nr05954a.
Muhr V, Wilhelm S, Hirsch T, Wolfbeis OS. Upconversion nanoparticles: from hydrophobic to hydrophilic surfaces. Acc Chem Res. 2014;47:3481–93. https://doi.org/10.1021/ar500253g.
Hassellöv M, Readman JW, Ranville JF, Tiede K. Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles. Ecotoxicology. 2008;17:344–61.
Love SA, Maurer-Jones MA, Thompson JW, et al. Assessing nanoparticle toxicity. Annu Rev Anal Chem. 2012;5:181–205. https://doi.org/10.1146/annurev-anchem-062011-143134.
Buchman JT, Hudson-Smith NV, Landy KM, Haynes CL. Understanding nanoparticle toxicity mechanisms to inform redesign strategies to reduce environmental impact. Acc Chem Res. 2019;52:1632–42. https://doi.org/10.1021/acs.accounts.9b00053.
Albanese A, Chan WCW. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano. 2011;5:5478–89. https://doi.org/10.1021/nn2007496.
Maurer-Jones MA, Lin YS, Haynes CL. Functional assessment of metal oxide nanoparticle toxicity in immune cells. ACS Nano. 2010;4:3363–73. https://doi.org/10.1021/nn9018834.
Maurer-Jones MA, Gunsolus IL, Murphy CJ, Haynes CL. Toxicity of engineered nanoparticles in the environment. Anal Chem. 2013;85:3036–49. https://doi.org/10.1021/ac303636s.
Kim HA, Lee BT, Na SY, et al. Characterization of silver nanoparticle aggregates using single particle-inductively coupled plasma-mass spectrometry (spICP-MS). Chemosphere. 2017;171:468–75. https://doi.org/10.1016/j.chemosphere.2016.12.063.
Perrault SD, Warren CWC. Synthesis and surface modification of highly monodispersed, spherical gold nanoparticles of 50−200 nm. J Am Chem Soc. 2009;131:17042–3. https://doi.org/10.1021/ja907069u.
Vigderman L, Zubarev ER. High-yield synthesis of gold nanorods with longitudinal SPR peak greater than 1200 nm using hydroquinone as a reducing agent. Chem Mater. 2013;25:1450–7. https://doi.org/10.1021/cm303661d.
Zhou S, Huo D, Goines S, et al. Enabling complete ligand exchange on the surface of gold nanocrystals through the deposition and then etching of silver. J Am Chem Soc. 2018;140:11898–901. https://doi.org/10.1021/jacs.8b06464.
Merrifield RC, Stephan C, Lead JR. Quantification of Au nanoparticle biouptake and distribution to freshwater algae using single cell - ICP-MS. Environ Sci Technol. 2018;52:2271–7. https://doi.org/10.1021/acs.est.7b04968.
Corte-Rodríguez M, Blanco-González E, Bettmer J, Montes-Bayón M. Quantitative analysis of transferrin receptor 1 (TfR1) in individual breast cancer cells by means of labeled antibodies and elemental (ICP-MS) detection. Anal Chem. 2019;91:15532–8. https://doi.org/10.1021/acs.analchem.9b03438.
Mavrakis E, Mavroudakis L, Lydakis-Simantiris N, Pergantis SA. Investigating the uptake of arsenate by Chlamydomonas reinhardtii cells and its effect on their lipid profile using single cell ICP-MS and easy ambient sonic-spray ionization-MS. Anal Chem. 2019;91:9590–8. https://doi.org/10.1021/acs.analchem.9b00917.
Cuello-Nuñez S, Abad-Álvaro I, Bartczak D, et al. The accurate determination of number concentration of inorganic nanoparticles using spICP-MS with the dynamic mass flow approach. J Anal At Spectrom. 2020. https://doi.org/10.1039/c9ja00415g.
Pace HE, Rogers NJ, Jarolimek C, et al. Determining transport efficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry. Anal Chem. 2011;83:9361–9. https://doi.org/10.1021/ac201952t.
Pace HE, Rogers NJ, Jarolimek C, et al. Single particle inductively coupled plasma-mass spectrometry: a performance evaluation and method comparison in the determination of nanoparticle size. Environ Sci Technol. 2012;46:12272–80. https://doi.org/10.1021/es301787d.
Kang H, Buchman JT, Rodriguez RS, et al. Stabilization of silver and gold nanoparticles: preservation and improvement of plasmonic functionalities. Chem Rev. 2019;119:664–99.
Muhammad Syed A, Sindhwani S, Wilhelm S, et al. Three-dimensional imaging of transparent tissues via metal nanoparticle labeling. J Am Chem Soc. 2017;139:9961–71. https://doi.org/10.1021/jacs.7b04022.
Haiss W, Thanh NTK, Aveyard J, Fernig DG. Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal Chem. 2007;79:4215–21. https://doi.org/10.1021/ac0702084.
Hineman A, Stephan C. Effect of dwell time on single particle inductively coupled plasma mass spectrometry data acquisition quality. J Anal At Spectrom. 2014;29:1252–7. https://doi.org/10.1039/c4ja00097h.
Lee S, Bi X, Reed RB, et al. Nanoparticle size detection limits by single particle ICP-MS for 40 elements. Environ Sci Technol. 2014;48:10291–300. https://doi.org/10.1021/es502422v.
Tan J, Yang Y, El Hadri H, et al. Fast quantification of nanorod geometry by DMA-spICP-MS. Analyst. 2019;144:2275–83. https://doi.org/10.1039/c8an02250j.
Kálomista I, Kéri A, Ungor D, et al. Dimensional characterization of gold nanorods by combining millisecond and microsecond temporal resolution single particle ICP-MS measurements. J Anal At Spectrom. 2017;32:2455–62. https://doi.org/10.1039/c7ja00306d.
Christau S, Moeller T, Genzer J, et al. Salt-induced aggregation of negatively charged gold nanoparticles confined in a polymer brush matrix. Macromolecules. 2017;50:7333–43. https://doi.org/10.1021/acs.macromol.7b00866.
Pamies R, Cifre JGH, Espín VF, et al. Aggregation behaviour of gold nanoparticles in saline aqueous media. J Nanopart Res. 2014;16. https://doi.org/10.1007/s11051-014-2376-4.
Kim T, Lee CH, Joo SW, Lee K. Kinetics of gold nanoparticle aggregation: experiments and modeling. J Colloid Interface Sci. 2008;318:238–43. https://doi.org/10.1016/j.jcis.2007.10.029.
Suk JS, Xu Q, Kim N, et al. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51.
Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine. 2011;6:715–28.
Manson J, Kumar D, Meenan BJ, Dixon D. Polyethylene glycol functionalized gold nanoparticles: the influence of capping density on stability in various media. Gold Bull. 2011;44:99–105. https://doi.org/10.1007/s13404-011-0015-8.
Zhang XD, Wu D, Shen X, et al. Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. Int J Nanomedicine. 2011;6:2071–81. https://doi.org/10.2147/ijn.s21657.
Walkey CD, Olsen JB, Guo H, et al. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc. 2012;134:2139–47. https://doi.org/10.1021/ja2084338.
Acknowledgments
The authors acknowledge assistance and fruitful discussions by Drs. S. Foster, R. Merrifield, C. Stephan, A. Madden P. Larson, R. Forester, H. Kirit, and PerkinElmer.
Funding
This work was supported in part by an NSF MRI grant (Award # 1828234), the IBEST/OUHSC Seed Grant for Interdisciplinary Research, and the Oklahoma Tobacco Settlement Endowment Trust awarded to the University of Oklahoma - Stephenson Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Oklahoma Tobacco Settlement Endowment Trust.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
ABC Highlights: authored by Rising Stars and Top Experts.
Electronic supplementary material
ESM 1
(PDF 895 kb)
Rights and permissions
About this article
Cite this article
Donahue, N.D., Francek, E.R., Kiyotake, E. et al. Assessing nanoparticle colloidal stability with single-particle inductively coupled plasma mass spectrometry (SP-ICP-MS). Anal Bioanal Chem 412, 5205–5216 (2020). https://doi.org/10.1007/s00216-020-02783-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02783-6