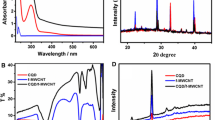
Abstract
The global occurrence of toxic hazards in aquatic ecosystems has aroused concern about the potential impacts on the ecological environment and human health in recent decades. Mercury(II) ions that originate from widespread sources including the mining industry, fossil fuel consumption, and industrial wastes are now well known as a highly toxic pollutant. Despite various detection methods which have been reported to sense Hg2+, it still poses a great challenge for us to develop a new effective sensing platform to replenish current fluorescent detection techniques. Here, we report a novel fluorescent biosensor using bamboo-like magnetic carbon nanotubes (BMCNTs) and FAM-labeled T-rich ssDNA for efficient detection of Hg2+ in aqueous solution. The proposed biosensor shows a good response toward Hg2+ detection over a linear response range of 0.05~1 μM (R2 = 0.98) with a detection limit of 20 nM. It also exhibits the capability to discriminate Hg2+ ions with negligible response to other metal ions, such as Ca2+, Cd2+, Cu2+, Mg2+, Mn2+, Ni2+, Pb2+, and Zn2+. Interestingly, the BMCNTs could be separated and recycled easily by using an external magnet, which means a much more cost-effective, easy-to-operate, and eco-friendly method for Hg2+ ion detection.






Similar content being viewed by others
References
Bi S, Ji B, Zhang Z, Zhu J-J. Metal ions triggered ligase activity for rolling circle amplification and its application in molecular logic gate operations. Chem Sci. 2013;4:1858–63.
Darbha GK, Singh AK, Rai US, Yu E, Yu H, Chandra RP. Selective detection of mercury (II) ion using nonlinear optical properties of gold nanoparticles. J Am Chem Soc. 2008;130:8038–43.
Wen S, Zeng T, Liu L, Zhao K, Zhao Y, Liu X, et al. Highly sensitive and selective DNA-based detection of mercury(II) with α-hemolysin nanopore. J Am Chem Soc. 2011;133:18312–7.
Kim HN, Ren WX, Kim JS, Yoon J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem Soc Rev. 2012;41:3210–44.
Bazzicalupi C, Caltagirone C, Cao Z, Chen Q, Di Natale C, Garau A, et al. Multimodal use of new coumarin-based fluorescent chemosensors: towards highly selective optical sensors for Hg2+ probing. Chem - A Eur J. 2013;19:14639–53.
Lin ZH, Zhu G, Zhou YS, Yang Y, Bai P, Chen J, et al. A self-powered triboelectric nanosensor for mercury ion detection. Angew Chem Int Ed. 2013;52:5065–9.
Yuan C-G, Lin K, Chang A. Determination of trace mercury in environmental samples by cold vapor atomic fluorescence spectrometry after cloud point extraction. Microchim Acta. 2010;171:313–9.
Yuan Y, Jiang S, Miao Q, Zhang J, Wang M, An L, et al. Fluorescent switch for fast and selective detection of mercury (II) ions in vitro and in living cells and a simple device for its removal. Talanta. 2014;125:204–9.
Chen G, Guo Z, Zeng G, Tang L. Fluorescent and colorimetric sensors for environmental mercury detection. Analyst. 2015;140:5400–43.
Nolan EM, Lippard SJ. Turn-on and ratiometric mercury sensing in water with a red-emitting probe. J Am Chem Soc. 2007;129:5910–8.
Chen P, He C. A general strategy to convert the MerR family proteins into highly sensitive and selective fluorescent biosensors for metal ions. J Am Chem Soc. 2004;126:728–9.
Kim E, Seo S, Seo ML, Jung JH. Functionalized monolayers on mesoporous silica and on titania nanoparticles for mercuric sensing. Analyst. 2010;135:149–56.
Du Y, Liu R, Liu B, Wang S, Han M-Y, Zhang Z. Surface-enhanced Raman scattering chip for femtomolar detection of mercuric ion (II) by ligand exchange. Anal Chem. 2013;85:3160–5.
Lu C, Jimmy Huang PJ, Ying Y, Liu J. Covalent linking DNA to graphene oxide and its comparison with physisorbed probes for Hg2+ detection. Biosens Bioelectron. 2016;79:244–50.
Wang P, Zhong R-B, Yuan M, Gong P, Zhao X-M, Zhang F. Mercury (II) detection by water-soluble photoluminescent ultra-small carbon dots synthesized from cherry tomatoes. Nucl Sci Tech. 2016;27:35.
Wang H, Liu Y, Liu G. Electrochemical biosensor using DNA embedded phosphorothioate modified RNA for mercury ion determination. ACS Sensors. 2018;3:624–31.
Zhang L, Li T, Li B, Li J, Wang E. Carbon nanotube-DNA hybrid fluorescent sensor for sensitive and selective detection of mercury(ii) ion. Chem Commun. 2010;46:1476–8.
Sadhukhan M, Barman S. Bottom-up fabrication of two-dimensional carbon nitride and highly sensitive electrochemical sensors for mercuric ions. J Mater Chem A. 2013;1:2752–6.
Bottini M, Mustelin T. Carbon materials: nanosynthesis by candlelight. Nat Nanotechnol. 2007;2:599.
Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56.
Kumar S, Rani R, Dilbaghi N, Tankeshwar K, Kim KH. Carbon nanotubes: a novel material for multifaceted applications in human healthcare. Chem Soc Rev. 2017;46:158–96.
Kumar S, Nehra M, Kedia D, Dilbaghi N, Tankeshwar K, Kim KH. Carbon nanotubes: a potential material for energy conversion and storage. Prog Energy Combust Sci. 2018;64:219–53.
Panwar N, Soehartono AM, Chan KK, Zeng S, Xu G, Qu J, et al. Nanocarbons for biology and medicine: sensing, imaging, and drug delivery. Chem Rev. 2019;119(16):9559–656.
Fiyadh SS, AlSaadi MA, Jaafar WZ, AlOmar MK, Fayaed SS, Mohd NS, et al. Review on heavy metal adsorption processes by carbon nanotubes. J Clean Prod. 2019;230:783–93.
Girishkumar G, Vinodgopal K, Kamat PV. Carbon nanostructures in portable fuel cells: single-walled carbon nanotube electrodes for methanol oxidation and oxygen reduction. J Phys Chem B. 2004;108:19960–6.
Kim TH, Lee J, Hong S. Highly selective environmental nanosensors based on anomalous response of carbon nanotube conductance to mercury ions. J Phys Chem C. 2009;113:19393–6.
Planeix JM, Coustel N, Coq B, Brotons V, Kumbhar PS, Dutartre R, et al. Application of carbon nanotubes as supports in heterogeneous catalysis. J Am Chem Soc. 1994;116:7935–6.
Mohamed RM, Abdel Salam M. Photocatalytic reduction of aqueous mercury(II) using multi-walled carbon nanotubes/Pd-ZnO nanocomposite. Mater Res Bull. 2014;50:85–90.
Ouyang R, Zhu Z, Tatum CE, Chambers JQ, Xue Z-L. Simultaneous stripping detection of Zn(II), Cd(II) and Pb(II) using a bimetallic Hg–Bi/single-walled carbon nanotubes composite electrode. J Electroanal Chem. 2011;656:78–84.
He L-L, Cheng L, Lin Y, Cui H-F, Hong N, Peng H, et al. A sensitive biosensor for mercury ions detection based on hairpin hindrance by thymine-Hg(II)-thymine structure. J Electroanal Chem. 2018;814:161–7.
Wu L-L, Wang Z, Zhao S-N, Meng X, Song X-Z, Feng J, et al. A metal–organic framework/DNA hybrid system as a novel fluorescent biosensor for mercury(II) ion detection. Chem – A Eur J. 2016;22:477–80.
Ge F, Li M-M, Ye H, Zhao B-X. Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J Hazard Mater. 2012;211–212:366–72.
Liu Z, Wang H, Liu C, Jiang Y, Yu G, Mu X, et al. Magnetic cellulose–chitosan hydrogels prepared from ionic liquids as reusable adsorbent for removal of heavy metal ions. Chem Commun. 2012;48:7350–2.
Malik R, Goyal A, Yadav S, Gupta N, Goel N, Kaushik A, et al. Functionalized magnetic nanomaterials for rapid and effective adsorptive removal of fluoroquinolones: comprehensive experimental cum computational investigations. J Hazard Mater. 2019;364:621–34.
Almomani F, Bhosale R, Khraisheh M, Kumar A, Almomani T. Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Appl Surf Sci. 2020;506:144924.
Deck CP, Vecchio K. Prediction of carbon nanotube growth success by the analysis of carbon–catalyst binary phase diagrams. Carbon. 2006;44:267–75.
Lin M, Tan JPY, Boothroyd C, Loh KP, Tok ES, Foo Y-L. Dynamical observation of bamboo-like carbon nanotube growth. Nano Lett. 2007;7:2234–8.
Song R, Jiang Z, Bi W, Cheng W, Lu J, Huang B, et al. The combined catalytic action of solid acids with nickel for the transformation of polypropylene into carbon nanotubes by pyrolysis. Chem – A Eur J. 2007;13:3234–40.
Cui X, Zhu L, Wu J, Hou Y, Wang P, Wang Z, et al. Fluorescent biosensor based on carbon dots-labeled oligodeoxyribonucleotide and graphene oxide for mercury (II) detection. Biosens Bioelectron. 2015;63:506–12.
He S, Song B, Li D, Zhu C, Qi W, Wen Y, et al. A graphene nanoprobe for rapid, sensitive, and multicolor fluorescent DNA analysis. Adv Funct Mater. 2010;20:453–9.
Tortolini C, Bollella P, Antonelli ML, Antiochia R, Mazzei F, Favero G. DNA-based biosensors for Hg2+ determination by polythymine–methylene blue modified electrodes. Biosens Bioelectron. 2015;67:524–31.
Chen G, Jin Y, Wang L, Deng J, Zhang C. Gold nanorods-based FRET assay for ultrasensitive detection of Hg2+. Chem Commun. 2011;47:12500–2.
Li M, Zhou X, Ding W, Guo S, Wu N. Fluorescent aptamer-functionalized graphene oxide biosensor for label-free detection of mercury (II). Biosens Bioelectron. 2013;41:889–93.
Pokhrel LR, Ettore N, Jacobs ZL, Zarr A, Weir MH, Scheuerman PR, et al. Novel carbon nanotube (CNT)-based ultrasensitive sensors for trace mercury(II) detection in water: a review. Sci Total Environ. 2017;574:1379–88.
Funding
This work was supported by the National Natural Science Foundation of China (no. 31772055).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 725 kb)
Rights and permissions
About this article
Cite this article
Jin, S., Wu, C., Ying, Y. et al. Magnetically separable and recyclable bamboo-like carbon nanotube–based FRET assay for sensitive and selective detection of Hg2+. Anal Bioanal Chem 412, 3779–3786 (2020). https://doi.org/10.1007/s00216-020-02631-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02631-7