Abstract
The scientific interest in gas sensors is continuously increasing because of their environmental, medical, industrial, and domestic applications. This has resulted in an increasing number of investigations being reported in the literature and communicated at conferences. The present review, organized in two parts, addresses the peculiarities of gas sensors based on mass-sensitive transducers, starting with their structure and functionality and progressing to implementation and specific use. In this first part of the review, we discuss the constructional peculiarities and operation regions and the physical and chemical processes governing the reception and transduction functions and the way in which they influence the sensor sensing parameters/features. Scientific outcomes and trends in research into gas sensors based on mass sensitive transducers are also considered.














Similar content being viewed by others
Notes
The transduction process is based on inertia and does not involve the gravitational field, even though it is usually present.
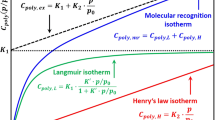
The examples given in Fig. 1 do not represent the state of the art regarding the characterization of GGSs, but represent the most used approaches.
Water vapor and oxygen are not only interfering gases/vapors but they can also deteriorate the receptor material.
The analyte-receptor interaction can also produce other effects (see “ The quality factor and influence of the receptor and analyte on resonant MSTs” and “Nongravimetric responses of bulk sorption receptors ”). Some of them are detected by the MST and can increase the sensor sensitivity and selectivity. Other are not directly detected by MSTs but can act as disturbing factors producing additional interferences.
The established terminology in the field addresses the odd multiples of the fundamental frequency as overtones and the even ones as harmonics.
This dashpot is fictitious and does not reflect a viscous friction as in the mechanical approaches to viscous fluids. It is a way to display the mechanical energy loss in irreversible thermodynamic processes only.
The assertion is almost correct for high-quality-factor (see “The quality factor and influence of the receptor and analyte on resonant MSTs”) TSMR transducers. If the quality factor is reduced by electrodes and/or sensing coatings, more accurate definitions are required.
Gas phase over critical isotherm.
Around the value for which the acoustoelectric attenuation has a maximum.
Because of material porosity, the thickness of the layer is an irrelevant parameter, so instead, the resonance frequency shift of the TSMR induced by the loading with the sensing layer is usually given (6 kHz6 kHz in this case).
In the case of high-Q crystals, the very small shift of the resonance frequency, spontaneously appearing to recover the “in-phase” (∆φ = 2nπ, where n is an integer) feedback, ensures the compensation of the damping. This frequency change is acceptable if it remains at the level of the noise.
The gas and liquid phases addressed here correspond to the mobile and stationary phases in chromatography.
The phase transformation occurs at constant temperature and its enthalpy expresses the whole exchanged heat. For a reversible phase transition the relation follows from the definition of entropy.
If the analyte-receptor “solution” can be regarded as a regular solution, then the mixing entropy will be, nevertheless, taken as zero.
Entropy of mixing is almost the same for a polymer family because of its configurational character.
The “catastrophe” means presumably negative values of the difference between the extrapolated glass-phase entropy and the solid-phase entropy (configurational entropy) below a given temperature, known as the “Kauzmann temperature.”
The model was named so by Grate later, not in the reference cited.
Lucklum et al. actually measured the complex reflection coefficient at the measuring cell connection.
The theoretical approach to this interaction, made in the frame of quantum mechanics, does not actually use the “induced-dipole” concept, which was introduced ad hoc to give a friendlier, classical perspective. Sometimes the London interaction is addressed as “van der Waals interaction,” which is not correct because the latter includes also other weak interactions.
For oxidizing gas the reaction path is different, involving an ionosorption process that competes with the oxygen one.
Drago et al. refer to Lewis acids and Lewis basis and not to the acid/base character of hydrogen bond discussed before.
Historically this parameter was determined on an empirical basis and symbolized by δ2.
References
Hulanicki A, Glab S, Ingman F. Chemical sensors: definitions and classification. Pure Appl Chem. 1991;63(9):1247–50.
Szulczyński B, Gębicki J. Currently commercially available chemical sensors employed for detection of volatile organic compounds in outdoor and indoor air. Environments. 2017;4(1):21.
King WH. Piezoelectric sorption detector. Anal Chem. 1964;36(9):1735–9.
Stanford Research Systems. Quartz Crystal Microbalance QCM200. https://www.thinksrs.com/products/qcm200.html
McNaught AD, Wilkinson A. Compendium of chemical terminology -IUPAC recommendations (IUPAC chemical data). 2nd ed. Wiley; 1997.
Currie LA. Nomenclature in evaluation of analytical methods including detection and quantification capabilities. Pure Appl Chem. 1995;67(10):1699–723.
Vessman J, Stefan RI, van Staden JF, Danzer K, Lindner W, Burns DT, et al. Selectivity in analytical chemistry (IUPAC recommendations 2001). Pure Appl Chem. 2001;73(8):1381–6.
Gauglitz G. Analytical evaluation of sensor measurements. Anal Bioanal Chem. 2018;410(1):5–13.
Ayad MM, Salahuddin NA, Minisy IM, Amer WA. Chitosan/polyaniline nanofibers coating on the quartz crystal microbalance electrode for gas sensing. Sens Actuators B. 2014;202:144–53. https://doi.org/10.1016/j.snb.2014.05.046.
Sayago I, Matatagui D, Fernández MJ, Fontecha JL, Jurewicz I, Garriga R, et al. Graphene oxide as sensitive layer in Love-wave surface acoustic wave sensors for the detection of chemical warfare agent simulants. Talanta. 2016;148:393–400. https://doi.org/10.1016/j.talanta.2015.10.069.
Gläser M, Borys M. Precision mass measurements. Rep Prog Phys. 2009;72(12):126101.
Curie J, Curie P. Développement, par pression, de l’électricité polaire dans les cristaux hémièdres à faces inclines. C R Hebd Seances Acad Sci. 1880;91:294–5.
Lippmann G. Principe de la conservation de l’électricité. Ann Chim Phys. 1881;24:145–78.
Bower AF. Applied mechanics of solids. Boca Raton: CRC Press; 2009.
Jackson JD. Classical electrodynamics. 3rd ed. Wiley; 1998.
Kittel C. Introduction to solid state physics. 8th ed. Wiley; 2004.
Ikeda T. Fundamentals of piezoelectricity. 1st ed. Oxford: Oxford University Press; 1990.
Rossetti GAJ. Thermodynamic theory. In: Heywang W, Lubitz K, Wersing W, editors. Piezoelectricity. Berlin: Springer; 2008. p. 293–515.
Devonshire AF. Theory of ferroelectrics. Adv Phys. 1954;3(10):85–130. https://doi.org/10.1080/00018735400101173.
Resta R. Electrical polarization and orbital magnetization: the modern theories. J Phys Condens Matter. 2010;22(12):123201.
Martin R. Piezoelectricity. Phys Rev B. 1972;5(4):1607–13. https://doi.org/10.1103/PhysRevB.5.1607.
Munn RW. Theory of piezoelectricity, electrostriction, and pyroelectricity in molecular crystals. J Chem Phys. 2010;132(10):104512. https://doi.org/10.1063/1.3340405.
Cohen RE. First-principles theories of piezoelectric materials. In: Heywang W, Lubitz K, Wersing W, editors. Piezoelectricity. Berlin: Springer; 2008. p. 471–92.
Shimamura K, Takeda H, Kohno T, Fukuda T. Growth and characterization of lanthanum gallium silicate La3Ga5SiO14 single crystals for piezoelectric applications. J Cryst Growth. 1996;163(4):388–92.
Fritze H, Tuller HL. Langasite for high-temperature bulk acoustic wave applications. Appl Phys Lett. 2001;78(7):976–7. https://doi.org/10.1063/1.1345797.
Sauerbrey G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z Phys. 1959;155(2):206–22. https://doi.org/10.1007/BF01337937.
Janata J. Principles of Chemical Sensors. second. Dordrecht, Heidelberg, London, New York: Springer; 2009. 373 p.
Barsan N, Gauglitz G, Oprea A, Ostertag E, Proll G, Rebner K, et al. Chemical and biochemical sensors, 1. Fundamentals. In: Ullmann’s encyclopedia of industrial chemistry. Weinheim: Wiley-VCH; 2016. p. 1–81. https://doi.org/10.1002/14356007.b06_121.pub2.
Lu C. In: Lu C, Czanderna AW, editors. Theory and practice of the quartz crystal microbalance. Amsterdam: Elsevier; 1984. p. 19–61.
Mecea VM. Is quartz crystal microbalance really a mass sensor? Sens Actuators A. 2006;128(2):270–7.
Ballantine DS, White RM, Martin SJ, Ricco AJ, Zellars ET, Frye GC, et al. Acoustic wave sensors - theory, design, and physico-chemical applications. San Diego: Elsevier. .
Johannsmann D. The quartz crystal microbalance in soft matter research. Cham: Springer. 2015. https://doi.org/10.1007/978-3-319-07836-6.
Butterworth S. On electrically-maintained vibrations. Proc Phys Soc Lond. 1914;27:410–24.
Van Dyke KS. The electric network equivalent of piezoelectric resonators. Phys Rev. 1925;25(6):895.
Van Dyke KS. The piezo-electric resonator and its equivalent network. Proc Inst Radio Eng. 1928;16:742–64.
Mason WP. Piezoelectric crystals and their applications to ultrasonics. 1st ed. New York: Van Nostrand; 1950.
Reed CE, Kanazawa KK, Kaufman JH. Physical description of a viscoelastically loaded AT-cut quartz resonator. J Appl Phys. 1990;68(5):1993–2001. https://doi.org/10.1063/1.346548.
Lucklum R, Hauptmann P. Transduction mechanism of acoustic wave based chemical and biochemical sensors. Meas Sci Technol. 2003;14(11):1854–64.
Martin SJ, Bandey HL, Cernosek RW, Hillman AR, Brown MJ. Equivalent-circuit model for the thickness-shear mode resonator with a viscoelastic film near film resonance. Anal Chem. 2000;72(1):141–9.
Janshoff A, Galla HJ, Steinem C. Piezoelectric mass-sensing devices as biosensors—an alternative to optical biosensors? Angew Chem Int Ed. 2010;32(6):4004.
Ushimi Y, Ito Y, Horiuchi H, Kadota M, Nozaki Y, Hotta Y, et al. Quartz crystal microbalance sensor for NH3 gas with compensation of humidity drift. Electron Commun Jpn. 2015;98(6):1–7. https://doi.org/10.1002/ecj.11653.
Rayleigh L. On waves propagated along the plane surface of an elastic solid. Proc Lond Math Soc. 1885;s1-17(1):4–11. https://doi.org/10.1112/plms/s1-17.1.4.
White RM, Voltmer FW. Direct piezoelectric coupling to surface elastic waves. Appl Phys Lett. 1965;7(12):314–6. https://doi.org/10.1063/1.1754276.
Wohltjen H, Dessy R. Surface acoustic wave probe for chemical analysis. I. Introduction and instrument description. Anal Chem. 1979;51(9):1458–64. https://doi.org/10.1021/ac50045a024.
Wohltjen H. Mechanism of operation and design considerations for surface acoustic wave device vapour sensors. Sensors Actuators. 1984;5(4):307–25.
Grate JW, Martin SJ, White RM. Acoustic wave microsensors. Anal Chem. 1993;65(21):940A–8A. https://doi.org/10.1021/ac00069a728.
Grate JW, Martin SJ, White RM. Acoustic wave microsensors part II. Anal Chem. 1993;65(22):987A–96A. https://doi.org/10.1021/ac00070a717.
Martin SJ, Ricco AJ, Niemczyk TM, Frye GC. Characterization of SH acoustic plate mode liquid sensors. Sensors Actuators. 1989;20(3):253–68.
Lamb H. On waves in an elastic plate. Proc R Soc A Math Phys Eng Sci. 1917;93(648):114–28. https://doi.org/10.1098/rspa.1917.0008.
Grate JW, Klusty M. Surface acoustic wave vapor sensors based on resonator devices. Anal Chem. 1991;63(17):1719–27. https://doi.org/10.1021/ac00017a013.
Caliendo C, Verardi P, Verona E, D’Amico A, Di Natale C, Saggio G, et al. Advances in SAW based gas sensors. Smart Mater Struct. 1997;6(6):689–99.
Gronewold TMA. Surface acoustic wave sensors in the bioanalytical field: recent trends and challenges. Anal Chim Acta. 2007;603(2):119–28.
Ben Youssef I, Alem H, Sarry F, Elmazria O, Jimenez Rioboo R, Arnal-Hérault C, et al. Functional poly(urethane-imide)s containing Lewis bases for SO2 detection by Love surface acoustic wave gas micro-sensors. Sensors Actuators B Chem. 2013;185:309–20. https://doi.org/10.1016/j.snb.2013.04.120.
Ippolito SJ, Kandasamy S, Kalantar-zadeh K, Wlodarski W, Galatsis K, Kiriakidis G, et al. Highly sensitive layered ZnO/LiNbO3 SAW device with InOx selective layer for NO2 and H2 gas sensing. Sensors Actuators B Chem. 2005;111(112):207–12. https://doi.org/10.1016/j.snb.2005.07.046.
Zheng P, Chin T-L, Greve D, Oppenheim I, Malone V, Ashok T, et al. Langasite SAW device with gas-sensitive layer. In: 2010 IEEE international ultrasonics symposium: IEEE; 2010. p. 1462–5.
Stoney GG. The tension of metallic films deposited by electrolysis. Proc R Soc A Math Phys Eng Sci. 1909;82(553):172–5. https://doi.org/10.1098/rspa.1909.0021.
Raiteri R, Grattarola M, Butt H-J, Skládal P. Micromechanical cantilever-based biosensors. Sensors Actuators B Chem. 2001;79(2–3):115–26. https://doi.org/10.1016/S0925-4005(01)00856-5.
Chen GY, Thundat T, Wachter EA, Warmack RJ. Adsorption-induced surface stress and its effects on resonance frequency of microcantilevers. J Appl Phys. 1995 Apr 15;77(8):3618–22 http://aip.scitation.org/doi/10.1063/1.359562.
Han SM, Benaroya H, Wei T. dynamics of transversely vibrating beams using four engineering theories. J Sound Vib. 1999;225(5):935–88.
Lange D, Hagleitner C, Hierlemann A, Brand O, Baltes H. Complementary metal oxide semiconductor cantilever arrays on a single chip: mass-sensitive detection of volatile organic compounds. Anal Chem. 2002;74(13):3084–95. https://doi.org/10.1021/ac011269j.
Yu H, Li X, Gan X, Liu Y, Liu X, Xu P, et al. Resonant-cantilever bio/chemical sensors with an integrated heater for both resonance exciting optimization and sensing repeatability enhancement. J Micromech Microeng. 2009;1(4):045023.
Lavrik NV, Sepaniak MJ, Datskos PG. Cantilever transducers as a platform for chemical and biological sensors. Rev Sci Instrum. 2004;75(7):2229–53.
Lavrik NV, Datskos PG. Femtogram mass detection using photothermally actuated nanomechanical resonators. Appl Phys Lett. 2003;82(16):2697–9. https://doi.org/10.1063/1.1569050.
Ilic B, Craighead HG, Krylov S, Senaratne W, Ober C, Neuzil P. Attogram detection using nanoelectromechanical oscillators. J Appl Phys. 2004;95(7):3694–703. https://doi.org/10.1063/1.1650542.
Yang YT, Callegari C, Feng XL, Ekinci KL, Roukes ML. Zeptogram-scale nanomechanical mass sensing. Nano Lett. 2006;6(4):583–6. https://doi.org/10.1021/nl052134m.
Ekinci KL, Yang YT, Roukes ML. Ultimate limits to inertial mass sensing based upon nanoelectromechanical systems. J Appl Phys. 2004;95(5):2682–9. https://doi.org/10.1063/1.1642738.
Ekinci KL, Roukes ML. Nanoelectromechanical systems. Rev Sci Instrum. 2005;76(6):061101. https://doi.org/10.1063/1.1927327.
Ferrari V, Lucklum R. Overview of acoustic-wave microsensors. In: Vives AA, editor. Piezoelectric transducers and applications. Berlin: Springer; 2009. p. 39–62. https://doi.org/10.1007/978-3-540-77508-9_2.
Abdolvand R, Bahreyni B, Lee JEY, Nabki F. Micromachined resonators: a review. Micromachines. 2016;7(9):160.
Rodahl M, Höök F, Krozer A, Brzezinski P, Kasemo B. Quartz crystal microbalance setup for frequency and Q-factor measurements in gaseous and liquid environments. Rev Sci Instrum. 1995;66(7):3924–30. https://doi.org/10.1063/1.1145396.
Galliou S, Goryachev M, Bourquin R, Abbé P, Aubry JP, Tobar ME. Extremely low loss phonontrapping cryogenic acoustic cavities for future physical experiments. Sci Rep. 2013;3(1):2132.
Blom FR. Dependence of the quality factor of micromachined silicon beam resonators on pressure and geometry. J Vac Sci Technol B. 1992;10(1):19. https://doi.org/10.1116/1.586300.
Uno T, Abe H, Miyamoto N, Jumonji H. Realization of miniature SAW resonators having high quality factor. Jpn J Appl Phys. 1981;20(S3):85.
Zhgoon S, Shvetsov A, Antcev I, Bogoslovsky S, Sapozhnikov G, Derkach M. Achieving ultimate Q-factors in SAW resonators on commercial LiNbO3 and LiTaO3 substrates. In: 2016 IEEE international ultrasonics symposium (IUS).: IEEE; 2016.
Mecea V, Bucur RV. The mechanism of the interaction of thin films with resonating quartz crystal substrates: the energy transfer model. Thin Solid Films. 1979;60(1):73–84.
Wenzel SW, White RM. Analytic comparison of the sensitivities of bulk-wave, surface-wave, and flexural plate-wave ultrasonic gravimetric sensors. Appl Phys Lett. 1989;54(20):1976–8. https://doi.org/10.1063/1.101189.
Martin SJ, Frye GC, Senturia SD. Dynamics and response of polymer-coated surface acoustic wave devices: effect of viscoelastic properties and film resonance. Anal Chem. 1994;66(14):2201–19. https://doi.org/10.1021/ac00086a003.
Lucklum R, Behling C, Hauptmann P. Role of mass accumulation and viscoelastic film properties for the response of acoustic-wave based chemical sensors. Anal Chem. 1999;71(13):2488–96.
Ferry JD. Viscoelastic properties of polymers. 1st ed. New York: Wiley; 1980.
Aklonis JJ, MacKnight WJ. Introduction to polymer viscoelasticity. 2nd ed. New York: Wiley; 1983.
Christiansen RM. Theory of viscoelasticity. 2nd ed. New York: Dover; 2010.
Kanazawa KK, Gordon JG. Frequency of a quartz microbalance in contact with liquid. Anal Chem. 1985;57(8):1770–1. https://doi.org/10.1021/ac00285a062.
Kanazawa KK, Gordon JG. The oscillation frequency of a quartz resonator in contact with liquid. Anal Chim Acta. 1985;175(C):99–105.
White CC, Schrag JL. Theoretical predictions for the mechanical response of a model quartz crystal microbalance to two viscoelastic media: a thin sample layer and surrounding bath medium. J Chem Phys. 1999;111(24):11192–206. https://doi.org/10.1063/1.480495.
Auld BA. Acoustic fields and waves in solids. 1st ed. New York: Wiley Interscience; 1973.
Tiersten HF, Sinha BK. A perturbation analysis of the attenuation and dispersion of surface waves. J Appl Phys. 1978;49(1):87–95. https://doi.org/10.1063/1.324340.
Johannsmann D. Derivation of the shear compliance of thin films on quartz resonators from comparison of the frequency shifts on different harmonics: a perturbation analysis. J Appl Phys. 2001;89(11):6356–64. https://doi.org/10.1063/1.1358317.
Martin SJ, Granstaff VE, Frye GC. Characterization of a quartz crystal microbalance with simultaneous mass and liquid loading. Anal Chem. 1991;63(20):2272–81. https://doi.org/10.1021/ac00020a015.
Voinova MV, Rodahl M, Jonson M, Kasemo B. Viscoelastic acoustic response of layered polymer films at fluid-solid interfaces: continuum mechanics approach. Phys Scr. 1999;59(5):391.
Hayward GL, Thompson M. A transverse shear model of a piezoelectric chemical sensor. J Appl Phys. 1998;83(4):2194–201. https://doi.org/10.1063/1.366956.
Benes E. Improved quartz crystal microbalance technique. J Appl Phys. 1984;56(3):608–26. https://doi.org/10.1063/1.333990.
Granstaff VE, Martin SJ. Characterization of a thickness-shear mode quartz resonator with multiple nonpiezoelectric layers. J Appl Phys. 1994;75(3):1319–29. https://doi.org/10.1063/1.356410.
Filiâtre C, Bardèche G, Valentin M. Transmission-line model for immersed quartz-crystal sensors. Sensors Actuators A. 1994;44(2):137–44.
Behling C, Lucklum R, Hauptmann P. Possibilities and limitations in quantitative determination of polymer shear parameters by TSM resonators. Sensors Actuators A. 1997;61(1–3):260–6.
Lucklum R, Hauptmann P. Transduction mechanism of acoustic wave based chemical and biochemical sensors. Meas Sci Technol. 2003;14(11):1854–64.
Kanazawa KK. Mechanical behaviour of films on the quartz microbalance. Faraday Discuss. 1997;107:77–90.
Lucklum R, Hauptmann P. Acoustic amplification. Response; 2000. p. 30–6.
Lucklum R, Hauptmann P. The quartz crystal microbalance: mass sensitivity, viscoelasticity and acoustic amplification. Sensors Actuators B Chem. 2000;70(1–3):30–6.
Grate JW, Zellers ET. The fractional free volume of the sorbed vapor in modeling the viscoelastic contribution to polymer-coated surface acoustic wave vapor sensor responses. Anal Chem. 2000;72(13):2861–8. https://doi.org/10.1021/ac991192n.
Lucklum R, Behling C, Hauptmann P. Gravimetric and non-gravimetric chemical quartz crystal resonators. Sensors Actuators B Chem. 2000;65(1–3):277–83. https://doi.org/10.1016/S09254005(99)00311-1.
Johannsmann D, Reviakine I, Rojas E, Gallego M. Effect of Sample Heterogeneity on the Interpretation of QCM(-D) Data: Comparison of Combined Quartz Crystal Microbalance/Atomic Force Microscopy Measurements with Finite Element Method Modelling. Anal Chem. 2008;80(23):8891–9 http://pubs.acs.org/doi/abs/10.1021/ac8013115.
Lucklum R, Hauptmann P. Acoustic microsensors—the challenge behind microgravimetry. Anal Bioanal Chem. 2006;384(3):667–82. https://doi.org/10.1007/s00216-005-0236-x.
Zhang D, Wang D, Zong X, Dong G, Zhang Y. High-performance QCM humidity sensor based on graphene oxide/tin oxide/polyaniline ternary nanocomposite prepared by in-situ oxidative polymerization method. Sensors Actuators B Chem. 2018;262:531–41.
Hierlemann A, Baltes H. CMOS-based chemical microsensors. Analyst. 2003;128(1):15–28.
Thomas S, Racs Z, Cole M, Gardner JW. High-frequency one-port Colpitts SAWoscillator for chemical sensing. In: Privman V, editor. Proceedings of the sixth international conference on advances in circuits, electronics and microelectronics (CENICS 2013). Wilmington: ThinkMind; 2013. p. 13–7.
Liu H, Zhang C, Weng Z, Guo Y, Wang Z. Resonance frequency readout circuit for a 900 MHz SAW device. Sensors. 2017;17(9):2131.
Voinova MV. On Mass loading and dissipation measured with acoustic wave sensors: a review. J Sensors. 2009;2009:1–13.
Ohlsson G, Langhammer C, Zorić I, Kasemo B. A nanocell for quartz crystal microbalance and quartz crystal microbalance with dissipation-monitoring sensing. Rev Sci Instrum. 2009;80(8):083905. https://doi.org/10.1063/1.3202207.
Esmeryan KD, Avramov ID, Radeva EI. Temperature behavior of solid polymer film coated quartz crystal microbalance for sensor applications. Sensors Actuators B Chem. 2015;216:240–6. https://doi.org/10.1016/j.snb.2015.04.034.
Qiao X, Zhang X, Tian Y, Meng Y. Progresses on the theory and application of quartz crystal microbalance. Appl Phys Rev. 2016;3(3):031106. https://doi.org/10.1063/1.4963312.
Hatch ER, Ballato A. Lateral-field excitation of quartz plates. In: 1983 ultrasonics symposium: IEEE; 1983. p. 512–5.
Chen Y-Y, Lai Y-T, Hsu C-H. Investigation of pseudo lateral field excited acoustic wave gas sensors with finite element method. Jpn J Appl Phys. 2014;53(7S):07KD01.
Kim J, Kim S, Ohashi T, Muramatsu H, Chang S-M, Kim W-S. Construction of simultaneous SPR and QCM sensing platform. Bioprocess Biosyst Eng. 2010;33(1):39–45. https://doi.org/10.1007/s00449-009-0370-5.
Ricco AJ, Martin SJ, Zipperian TE. Surface acoustic wave gas sensor based on film conductivity changes. Sensors Actuators. 1985;8(4):319–33.
Jakubik W. Elemental theory of a SAW gas sensor based on electrical conductivity changes in bi-layer nanostructures. Sensors Actuators B Chem. 2014;203:511–6.
Jakubik W, Wrotniak J, Powroźnik P. Theoretical analysis of a surface acoustic wave gas sensor mechanism using electrical conductive bi-layer nanostructures. Sensors Actuators B Chem. 2018;262:947–52.
Barié N, Bücking M, Stahl U, Rapp M. Detection of coffee flavor ageing by solid-phase microextraction/surface acoustic wave sensor array technique (SPME/SAW). Food Chem. 2015;176:212–8.
Jin X, Huang Y, Mason A, Zeng X. Multichannel monolithic quartz crystal microbalance gas sensor array. Anal Chem. 2009;81(2):595–603. https://doi.org/10.1021/ac8018697.
Rabe J, Buttgenbach S, Schroder J, Hauptmann P. Monolithic miniaturized quartz microbalance array and its application to chemical sensor systems for liquids. IEEE Sensors J. 2003;3(4):361–8.
Tao W, Lin P, Ai Y, Wang H, Ke S, Zeng X. Multichannel quartz crystal microbalance array: fabrication, evaluation, application in biomarker detection. Anal Biochem. 2016;494:85–92. https://doi.org/10.1016/j.ab.2015.11.001.
Yan XF, Li DM, Hou CC, Wang X, Zhou W, Liu M, et al. Comparison of response towards NO2 and H2S of PPy and PPy/TiO2 as SAW sensitive films. Sensors Actuators B Chem. 2012;161(1):329–33.
Speller NC, Siraj N, Regmi BP, Marzoughi H, Neal C, Warner IM. Rational design of QCM-D virtual sensor arrays based on film thickness, viscoelasticity, and harmonics for vapor discrimination. Anal Chem. 2015;87(10):5156–66. https://doi.org/10.1021/ac5046824.
Speller NC, Siraj N, Vaughan S, Speller LN, Warner IM. Assessment of QCM array schemes for mixture identification: citrus scented odors. RSC Adv. 2016;6(98):95378–86. https://doi.org/10.1039/C6RA16988K.
Eichelbaum F, Borngräber R, Schröder J, Lucklum R, Hauptmann P. Interface circuits for quartz-crystal-microbalance sensors. Rev Sci Instrum. 1999;70(5):2537–45. https://doi.org/10.1063/1.1149788.
Lucklum R, Eichelbaum F. Interface circuits for QCMsensors. In: Steinem C, Janshoff A, editors. Piezoelectric sensors. Berlin: Springer; 2007. p. 3–47. https://doi.org/10.1007/5346_023.
Arnau A. A review of interface electronic systems for AT-cut quartz crystal microbalance applications in liquids. Sensors. 2008;8(1):370–411.
Alassi A, Benammar M, Brett D. Quartz crystal microbalance electronic interfacing systems: a review. Sensors. 2017;17(12):2799.
Peschel A, Langhoff A, Johannsmann D. Coupled resonances allow studying the aging of adhesive contacts between a QCM surface and single, micrometer-sized particles. Nanotechnology. 2015;26(48):484001. https://doi.org/10.1088/0957-4484/26/48/484001.
Furusawa H, Sekine T, Ozeki T. Hydration and viscoelastic properties of high- and low-density polymer brushes using a quartz crystal microbalance based on admittance analysis (QCM-A). Macromolecules. 2016;49(9):3463–70. https://doi.org/10.1021/acs.macromol.6b00035.
Barnes C. Development of quartz crystal oscillators for under liquid sensing. Sensors Actuators A. 1991;29(1):59–69.
Mills CA, Chai KTC, Milgrew MJ, Glidle A, Cooper JM, Cumming DRS. A Multiplexed impedance analyzer for characterizing polymer-coated QCM sensor arrays. IEEE Sensors J. 2006;6(4):996–1002.
Wudy F, Multerer M, Stock C, Schmeer G, Gores HJ. Rapid impedance scanning QCM for electrochemical applications based on miniaturized hardware and high-performance curve fitting. Electrochim Acta. 2008;53(22):6568–74.
Rodahl M, Kasemo B. A simple setup to simultaneously measure the resonant frequency and the absolute dissipation factor of a quartz crystal microbalance. Rev Sci Instrum. 1996;67(9):3238–41. https://doi.org/10.1063/1.1147494.
Edvardsson M, Rodahl M, Kasemo B, Hook F. A dual-frequency QCM-D setup operating at elevated oscillation amplitudes. Anal Chem. 2005;77(15):4918–26.
Baù M, Ferrari M, Ferrari V. Analysis and validation of contactless time-gated interrogation technique for quartz resonator sensors. Sensors (Basel). 2017;17(6):1264.
Beißner S, Thies J-W, Bechthold C, Kuhn P, Thürmann B, Dübel S, et al. Low-cost, in-liquid measuring system using a novel compact oscillation circuit and quartz-crystal microbalances (QCMs) as a versatile biosensor platform. J Sens Sens Syst. 2017;6(2):341–50.
Tumurbaatar B, Kim M-J, Park C-H, Kim CS. A portable and computer-simulation analysis for the real-time measurement of the QCMD systems for the biomedical application. Sens Biosens Res. 2018;21:75–81.
Baù M, Tonoli E, Ferrari V, Marioli D. Contactless electromagnetic switched interrogation of micromechanical cantilever resonators. Sensors Actuators A. 2011;172(1):195–203.
Ferrari V, Marioli D, Taroni A. Improving the accuracy and operating range of quartz microbalance sensors by a purposely designed oscillator circuit. IEEE Trans Instrum Meas. 2001;50(5):1119–22.
Arnau A, Sogorb T, Jiménez Y. Circuit for continuous motional series resonant frequency and motional resistance monitoring of quartz crystal resonators by parallel capacitance compensation. Rev Sci Instrum. 2002;73(7):2724. https://doi.org/10.1063/1.1484254.
Sell JK, Niedermayer AO, Jakoby B. Digital phase-locked loop circuit for driving resonant sensors. Procedia Eng. 2010;5:204–7. https://doi.org/10.1016/j.proeng.2010.09.083.
Hu Z, Hedley J, Keegan N, Spoors J, Gallacher B, McNeil C. One port electronic detection strategies for improving sensitivity in piezoelectric resonant sensor measurements. Sensors. 2016;16(11):1781.
Montagut Y, García JV, Jiménez Y, March C, Montoya Á, Arnau A. Validation of a phase-mass characterization concept and interface for acoustic biosensors. Sensors. 2011;11(5):4702–20.
Arnau A, Montagut Y, García JV, Jiménez Y. A different point of view on the sensitivity of quartz crystal microbalance sensors. Meas Sci Technol. 2009;20(12):124004.
Yurish S. Low-cost, intelligent data acquisition system for QCM and other resonator-based bio- and chemical sensors. Int J Comput. 2008;7(2):9–17.
Yurish SY, Kirianaki NV, Pallas-Areny R. Universal frequency-to-digital converter for quasidigital and smart sensors: specifications and applications. Sens Rev. 2005;25(2):92–9.
Dunham GC, Benson NH, Petelenz D, Janata J. Dual quartz crystal microbalance. Anal Chem. 1995;67(2):267–72. https://doi.org/10.1021/ac00098a005.
Ito T, Fujii Y, Yamanishi N, Asai N, Shimizu T. Electrodeposited ZnO thin film on twin sensor QCM for sensing of ethanol at room temperature. Procedia Eng. 2016;168:411–4. https://doi.org/10.1016/j.proeng.2016.11.192.
Muckley ES, Anazagasty C, Jacobs CB, Hianik T, Ivanov IN. Low-cost scalable quartz crystal microbalance array for environmental sensing. Proc SPIE. 2016;9944:99440Y.
Klinkhachorn P, Huner B, Overton EB, Dharmasena HP, Gustowski DA. A Microprocessor based piezoelectric quartz microbalance system for compound-specific detection. IEEE Trans Instrum Meas. 1990;39(1):264–8.
Singh HK, Bezboruah T. Micro-controller based frequency to digital converter for interfacing frequency output sensors. In: 2015 international conference on electronic design, computer networks & automated verification (EDCAV): IEEE; 2015. p. 34–7.
Colodro F, Torralba A. Frequency-to-digital conversion based on a sampled phase-locked loop. Microelectron J. 2013;44(10):880–7. https://doi.org/10.1016/j.mejo.2013.02.003.
International Frequency Sensor Association. Universal frequency-to-digital converter (UFDC1). Specification and application note. 2010; http://www.sensorsportal.com/DOWNLOADS/UFDC_1.pdf.
Yurish S. A Simple and universal resistive-bridge sensors interface. Sensors Transducers. 2011;10:45–59.
Beeley JM, Mills C, Hammond PA, Glidle A, Cooper JM, Wang L, et al. All-digital interface ASIC for a QCM-based electronic nose. Sensors Actuators B Chem. 2004;103(1–2):31–6.
Hyunsoo Kim JT, Lim J, Choi K, Kenny D. Direct mounting of quartz crystal on a CMOS PLL chip. In: Proceedings of the 2004 IEEE international frequency control symposium and exposition, 2004: IEEE; 2004. p. 165–8.
Karasek FW, Gibbins KR. A gas chromatograph based on the piezoelectric detector. J Chromatogr Sci. 1971;9(9):535–40.
Chen D, Sun X, Zhang K, Fan G, Wang Y, Li G, et al. A noncontact dibutyl phthalate sensor based on a wireless-electrodeless QCM-D modified with nano-structured nickel hydroxide. Sensors. 2017;17(7):1681.
Chen D, Zhang K, Zhou H, Fan G, Wang Y, Li G, et al. A wireless electrodeless quartz crystal microbalance with dissipation DMMP sensor. Sensors Actuators B Chem. 2018;261:408–17. https://doi.org/10.1016/j.snb.2018.01.105.
Atkins P, de Paula J. Atkins’ physical chemistry. 10th ed. Oxford: Oxford University Press; 2014.
Haug M, Schierbaum KD, Gauglitz G, Göpel W. Chemical sensors based upon polysiloxanes: comparison between optical, quartz microbalance, calorimetric, and capacitance sensors. Sensors Actuators B Chem. 1993;11(1–3):383–91.
Shutler PME, Cheah HM. Applying Boltzmann’s definition of entropy. Eur J Phys. 1998;19(4):371–7.
Barton AFM. Solubility parameters. Chem Rev. 1975;75(6):731–53. https://doi.org/10.1021/cr60298a003.
Huggins ML. Solutions of long chain compounds. J Chem Phys. 1941;9(5):440. https://doi.org/10.1063/1.1750930.
Flory P. Thermodynamics of dilute solutions of high polymers. J Chem Phys. 1945;13(11):453–65. https://doi.org/10.1063/1.1723978.
Flory PJ. The thermodynamics of polymer solutions. Princ Polym Chem. 1953:50–1.
Hierlemann A, Ricco AJ, Bodenhöfer K, Dominik A, Göpel W. Conferring selectivity to chemical sensors via polymer side-chain selection: thermodynamics of vapor sorption by a set of polysiloxanes on thickness-shear mode resonators. Anal Chem. 2000;72(16):3696–708. https://doi.org/10.1021/ac991298i.
Hierlemann A, Lange D, Hagleitner C, Kerness N, Koll A, Brand O, et al. Application-specific sensor systems based on CMOS chemical microsensors. Sensors Actuators B Chem. 2000;70(1–3):2–11.
Grate JW, Snow A, Ballantine DS, Wohltjen H, Abraham MH, Mcgill RA, et al. Determination of partition coefficients from surface acoustic wave vapor sensor responses and correlation with gas–liquid chromatographic partition coefficients. Anal Chem. 1988;60(9):869–75. https://doi.org/10.1021/ac00160a010.
Bodenhöfer K, Hierlemann A, Noetzel G, Weimar U, Göpel W. Performances of mass-sensitive devices for gas sensing: thickness shear mode and surface acoustic wave transducers. Anal Chem. 1996;68(13):2210–8. https://doi.org/10.1021/ac9600215.
Grate JW, Kaganove SN. Comparisons of polymer/gas partition coefficients calculated from responses of thickness shear mode and surface acoustic wave vapor sensors. Anal Chem. 1998;70(1):199–203. https://doi.org/10.1021/ac970608z.
Fox TG. Influence of diluent and of copolymer composition on the glass transition temperature of a polymer system. Bull Am Phys Soc. 1956;1:123.
Pei J, Zhang JS. Determination of adsorption isotherm and diffusion coefficient of toluene on activated carbon at low concentrations. Build Environ. 2012;48:66–76.
El Sabahy J, Berthier J, Bonnet L, Matheron M, Bordy T, Yeromonahos C, et al. Tolueneorganic thin films partition coefficients analyzed with Langmuir adsorption theory and finite elements simulations. Sensors Actuators B Chem. 2014;202:941–8. https://doi.org/10.1016/j.snb.2014.05.041.
Şen Z, Tarakci DK, Gürol I, Ahsen V, Harbeck M. Governing the sorption and sensing properties of titanium phthalocyanines by means of axial ligands. Sensors Actuators B Chem. 2016;229:581–6.
Fu Y, Finklea HO. Quartz crystal microbalance sensor for organic vapor detection based on molecularly imprinted polymers. Anal Chem. 2003;75(20):5387–93. https://doi.org/10.1021/ac034523b.
Zhang K, Hu R, Fan G, Li G. Graphene oxide/chitosan nanocomposite coated quartz crystal microbalance sensor for detection of amine vapors. Sensors Actuators B Chem. 2017;243:721–30. https://doi.org/10.1016/j.snb.2016.12.063.
El Sabahy J, Berthier J, Ricoul F, Jousseaume V. Toward optimized SiOCH films for BTEX detection: impact of chemical composition on toluene adsorption. Sensors Actuators B Chem 2018;258:628–636. doi:https://doi.org/10.1016/j.snb.2017.11.105.
Doolittle AK. Studies in Newtonian flow. II. The dependence of the viscosity of liquids on freespace. J Appl Phys. 1951;22(12):1471–5. https://doi.org/10.1063/1.1699894.
Fox TG, Flory PJ. Viscosity–molecular weight and viscosity–temperature relationships for polystyrene and polyisobutylene. J Am Chem Soc. 1948;70(7):2384–95. https://doi.org/10.1021/ja01187a021.
Fox TG, Flory PJ. Second-order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J Appl Phys. 1950;21(6):581–91. https://doi.org/10.1063/1.1699711.
Fox TG, Flory PJ. Further studies on the melt viscosity of polyisobutylene. J Phys Chem. 1951;55(2):221–34. https://doi.org/10.1021/j150485a010.
Fox TG, Flory PJ. The glass temperature and related properties of polystyrene. Influence of molecular weight. J Polym Sci. 1954;14(75):315–9. https://doi.org/10.1002/pol.1954.120147514.
Williams ML, Landel RF, Ferry JD. The temperature dependence of relaxation mechanisms in amorphous polymers and other glass-forming liquids. J Am Chem Soc. 1955;77(14):3701–7. https://doi.org/10.1021/ja01619a008.
Fujita H, Kishimoto A. Diffusion-controlled stress relaxation in polymers. II. Stress relaxation in swollen polymers. J Polym Sci. 1958;28(118):547–67. https://doi.org/10.1002/pol.1958.1202811806.
Ferry JD, Stratton RA. The free volume interpretation of the dependence of viscosities and viscoelastic relaxation times on concentration, pressure, and tensile strain. Kolloid Z. 1960;171(2):107–11. https://doi.org/10.1007/BF01520041.
Wang TT, Matsuoka S. The free volume concept and its implications on dilation in glassy polymers under shear stresses. J Polym Sci Polym Lett Ed. 1980;18(9):593–8. https://doi.org/10.1002/pol.1980.130180902.
McKenna GB. Glass Formation and glassy behavior. In: Comprehensive polymer science and supplements: Elsevier; 1989. p. 311–62.
White RP, Lipson JEG. Polymer free volume and its connection to the glass transition. Macromolecules. 2016;49(11):3987–4007. https://doi.org/10.1021/acs.macromol.6b00215.
Kauzmann W. The nature of the glassy state and the behavior of liquids at low temperatures. Chem Rev. 1948;43(2):219–56. https://doi.org/10.1021/cr60135a002.
Gibbs JH, DiMarzio EA. Nature of the glass transition and the glassy state. J Chem Phys. 1958;28(3):373–83. https://doi.org/10.1063/1.1744141.
DiMarzio EA, Gibbs JH. Chain stiffness and the lattice theory of polymer phases. J Chem Phys. 1958;28(5):807–13. https://doi.org/10.1063/1.1744275.
Adam G, Gibbs JH. On the temperature dependence of cooperative relaxation properties in glass-forming liquids. J Chem Phys. 1965;43(1):139–46. https://doi.org/10.1063/1.1696442.
Donth E-J. Relaxation and thermodynamics in polymers glass transition. Berlin: Akademie; 1992.
Debenedetti PG, Stillinger FH. Supercooled liquids and the glass transition. Nature. 2001;410:259. https://doi.org/10.1038/35065704.
Stillinger FH, Debenedetti PG. Glass transition thermodynamics and kinetics. Annu Rev Condens Matter Phys. 2013;4(1):263–85. https://doi.org/10.1146/annurev-conmatphys-030212-184329.
Flory PJ. Principles of polymer chemistry. Ithaca: Cornell University Press; 1953.
Biesalski M, Rühe J. Swelling of a polyelectrolyte brush in humid air. Langmuir. 2000;16(4):1943–50. https://doi.org/10.1021/la990863+.
Altenberend U, Oprea A, Barsan N, Weimar U. Contribution of polymeric swelling to the overall response of capacitive gas sensors. Anal Bioanal Chem. 2013;405(20):6445–52.
Domack A, Prucker O, Rühe J, Johannsmann D. Swelling of a polymer brush probed with a quartz crystal resonator. Phys Rev E. 1997;56(1):680–9. https://doi.org/10.1103/PhysRevE.56.680.
Martin SJ, Frye GC. Surface acoustic wave response to changes in viscoelastic film properties. Appl Phys Lett. 1990;57(18):1867–9.
Grate JW, Klusty M, McGill RA, Abraham MH, Whiting G, Andonian-Haftvan J. The predominant role of swelling-induced modulus changes of the sorbent phase in determining the responses of polymer-coated surface acoustic wave vapor sensors. Anal Chem. 1992;64(6):610–24. https://doi.org/10.1021/ac00030a009.
Grate JW, Kaganove SN, Bhethanabotla VR. Examination of mass and modulus contributions to thickness shear mode and surface acoustic wave vapour sensor responses using partition coefficients. Faraday Discuss. 1997;107:259–83.
Johannsmann D, Mathauer K, Wegner G, Knoll W. Viscoelastic properties of thin films probed with a quartz-crystal resonator. Phys Rev B. 1992;46(12):7808–15. https://doi.org/10.1103/PhysRevB.46.7808.
Lucklum R, Behling C, Cernosek RW, Martin SJ. Determination of complex shear modulus with thickness shear mode resonators. J Phys D Appl Phys. 1997;30(3):346–56.
Sahm M, Oprea A, Bârsan N, Weimar U. Interdependence of ammonia and water sorption in polyacrylic acid layers. Sensors Actuators B Chem. 2008;130(1):502–7.
Hoerter M, Oprea A, Bârsan N, Weimar U. Chemical interaction of gaseous ammonia and water vapour with polyacrylic acid layers. Sensors Actuators B Chem. 2008;134(2):743–9.
Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. 1918;40(9):1361–403. https://doi.org/10.1021/ja02242a004.
Langmuir I. Nobel Lecture: surface chemistry. 1932. http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1932/langmuirlecture.html. Accessed 29 Jul 2018.
Kabir KMM, Sabri YM, Myers L, Harrison I, Boom E, Coyle VE, et al. Investigating the crossinterference effects of alumina refinery process gas species on a SAW based mercury vapor sensor. Hydrometallurgy. 2017;170:51–7. https://doi.org/10.1016/j.hydromet.2016.05.015.
Lv Y, Xu P, Yu H, Xu J, Li X. Ni-MOF-74 as sensing material for resonant-gravimetric detection of ppb-level CO. Sensors Actuators B Chem. 2018;262:562–9.
Darwish HMB, Okur S. CO adsorption kinetics of ferrocene-conjugated polypyrrole using quartz microbalance technique. Sensors Actuators B Chem. 2014;200:325–31. https://doi.org/10.1016/j.snb.2014.03.107.
Wang L, Zhu Y, Xiang Q, Cheng Z, Chen Y, Xu J. One novel humidity-resistance formaldehyde molecular probe based hydrophobic diphenyl sulfone urea dry-gel: synthesis, sensing performance and mechanism. Sensors Actuators B Chem. 2017;251:590–600. https://doi.org/10.1016/j.snb.2017.05.074.
Ogimoto Y, Selyanchyn R, Takahara N, Wakamatsu S, Lee S-W. Detection of ammonia in human breath using quartz crystal microbalance sensors with functionalized mesoporous SiO2 nanoparticle films. Sensors Actuators B Chem. 2015;215:428–36.
Wang X, Ding B, Yu J, Si Y, Yang S, Sun G. Electro-netting: fabrication of two-dimensional nano-nets for highly sensitive trimethylamine sensing. Nanoscale. 2011;3(3):911–5.
Tokura Y, Nakada G, Moriyama Y, Oaki Y, Imai H, Shiratori S. Ultrasensitive detection of methylmercaptan gas using layered manganese oxide nanosheets with a quartz crystal microbalance sensor. Anal Chem. 2017;89(22):12123–30. https://doi.org/10.1021/acs.analchem.7b02738.
Matsuguchi M, Harada N, Omori S. Poly(N-isopropylacrylamide) nanoparticles for QCM-based gas sensing of HCl. Sensors Actuators B Chem. 2014;190:446–50.
Matsuguchi M, Takaoka K, Kai H. HCl gas adsorption/desorption properties of poly(Nisopropylacrylamide) brushes grafted onto quartz resonator for gas-sensing applications. Sensors Actuators B Chem. 2015;208:106–11.
Jia Y, Yu H, Cai J, Li Z, Dong F. Explore on the quantitative analysis of specific surface area on sensitivity of polyacrylic acid-based QCM ammonia sensor. Sensors Actuators B Chem. 2017;243:1042–5. https://doi.org/10.1016/j.snb.2016.12.090.
Zheng Q, Zhu Y, Xu J, Cheng Z, Li H, Li X. Fluoroalcohol and fluorinated-phenol derivatives functionalized mesoporous SBA-15 hybrids: high-performance gas sensing toward nerve agent. J Mater Chem. 2012;22(5):2263–70.
Li H, Zheng Q, Luo J, Cheng Z, Xu J. Impacts of meso-structure and organic loadings of fluoroalcohol derivatives/SBA-15 hybrids on nerve agent simulant sensing. Sensors Actuators B Chem. 2013;187:604–10.
Khanniche S, Mathieu D, Pereira F, Frenois C, Colin D, Barthet C, et al. Quantitative evaluation of the responses of a gravimetric gas sensor based on mesoporous functionalized silica: application to 2,4-DNT and TNT detection. Sensors Actuators B Chem. 2017;248:470. https://doi.org/10.1016/j.snb.2017.03.137.
Tai H, Zhen Y, Liu C, Ye Z, Xie G, Du X, et al. Facile development of high performance QCM humidity sensor based on protonated polyethylenimine-graphene oxide nanocomposite thin film. Sensors Actuators B Chem. 2016;230:501–9.
Bayram A, Özbek C, Şenel M, Okur S. CO gas sorption properties of ferrocene branched chitosan derivatives. Sensors Actuators B Chem. 2017;241:308–13.
Zhang D, Wang D, Zong X, Dong G, Zhang Y. High-performance QCM humidity sensor based on graphene oxide/tin oxide/polyaniline ternary nanocomposite prepared by in-situ oxidative polymerization method. Sensors Actuators B Chem. 2018;262:531–41.
Hirai K, Sumida K, Meilikhov M, Louvain N, Nakahama M, Uehara H, et al. Impact of crystal orientation on the adsorption kinetics of a porous coordination polymer–quartz crystal microbalance hybrid sensor. J Mater Chem C. 2014;2(17):3336.
Barton AFM. Handbook of solubility parameters and other cohesion parameters. 2nd ed. Boca Raton: CRC Press; 1991.
Steiner T. The Hydrogen bond in the solid state. Angew Chem Int Ed. 2002;41(1):48–76. https://doi.org/10.1002/1521-3773%2820020104%2941%3A1%3C48%3A%3AAIDANIE48%3E3.0.CO%3B2-U229.
Desiraju GR. The C-H O hydrogen bond: structural implications and supramolecular design. Acc Chem Res. 1996;29(9):441–9. https://doi.org/10.1021/ar950135n.
Yan D, Xu P, Xiang Q, Mou H, Xu J, Wen W, et al. Polydopamine nanotubes: bio-inspired synthesis, formaldehyde sensing properties and thermodynamic investigation. J Mater Chem A. 2016;4(9):3487–93.
Xu X, Li C, Pei K, Zhao K, Zhao Z, Li H. Ionic liquids used as QCM coating materials for the detection of alcohols. Sensors Actuators B Chem. 2008;134(1):258–65.
Grate JW, Abraham MH. Solubility interactions and the design of chemically selective sorbent coatings for chemical sensors and arrays. Sensors Actuators B Chem. 1991;3(2):85–111.
Hunter CA, Sanders JKM. The Nature of π-π Interactions. J Am Chem Soc. 1990;112(14):5525–34. https://doi.org/10.1021/ja00170a016.
Bloom JWG, Wheeler SE. Taking the aromaticity out of aromatic interactions. Angew Chem Int Ed. 2011;50(34):7847–9. https://doi.org/10.1002/anie.201102982.
Kumar A, Brunet J, Varenne C, Ndiaye A, Pauly A. Phthalocyanines based QCM sensors for aromatic hydrocarbons monitoring: role of metal atoms and substituents on response to toluene. Sensors Actuators B Chem. 2016;230:320–9. https://doi.org/10.1016/j.snb.2016.02.032.
Li H-Y, Chu Y-H. Exploiting solvate ionic liquids for amine gas analysis on a quartz crystal microbalance. Anal Chem. 2017;89(10):5186–92. https://doi.org/10.1021/acs.analchem.7b00857.
Barsan N, Weimar U. Conduction model of metal oxide gas sensors. J Electroceram. 2001;7(3):143–67. https://doi.org/10.1023/A:1014405811371.
Buckingham AD, Fowler PW, Hutson JM. Theoretical studies of van der Waals molecules and intermolecular forces. Chem Rev. 1988;88(6):963–88. https://doi.org/10.1021/cr00088a008.
Chalasinski G, Gutowski M. Weak interactions between small systems. Models for studying the nature of intermolecular forces and challenging problems for ab initio calculations. Chem Rev. 1988;88(6):943–62. https://doi.org/10.1021/cr00088a007.
Koch U, Popelier PLA. Characterization of C-H-O hydrogen bonds on the basis of the charge density. J Phys Chem. 1995;99(24):9747–54. https://doi.org/10.1021/j100024a016.
Hansen CM. The three dimensional solubility parameter and solvent diffusion coefficient. In: Their importance in surface coating formulation. Copenhagen: Danish Technical Press; 1967. https://www.hansen-solubility.com/contents/HSP1967-OCR.pdf.
Drago RS, Vogel GC, Needham TE. Four-parameter equation for predicting enthalpies of adduct formation. J Am Chem Soc. 1971;93(23):6014–26. https://doi.org/10.1021/ja00752a010.
Kamlet MJ, Abboud JLM, Abraham MH, Taft RW. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters, π*, α, and β, and some methods for simplifying the generalized solvatochromic equation. J Organomet Chem. 1983;48(17):2877–87. https://doi.org/10.1021/jo00165a018.
Markham A, Kobe KA. The solubility of gases in liquids. Chem Rev. 1941;28(3):519–88. https://doi.org/10.1021/cr60091a003.
Hierlernann A, Zellers ET, Ricco AJ. Use of linear solvation energy relationships for modeling responses from polymer-coated acoustic-wave vapor sensors. Anal Chem. 2001;73(14):3458–66. https://doi.org/10.1021/ac010083h.
Harbeck M, Şen Z, Gümüş G, Gürol I, Musluoǧlu E, Öztürk ZZ, et al. Customized vic-dioximes and their metal complexes for enhanced chemical sensing of polar organic molecules. Sensors Actuators B Chem. 2013;188:1004–11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Research involving humans and/or animals
The article is a review and involved no humans or animals in investigations performed by the authors themselves.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oprea, A., Weimar, U. Gas sensors based on mass-sensitive transducers part 1: transducers and receptors—basic understanding. Anal Bioanal Chem 411, 1761–1787 (2019). https://doi.org/10.1007/s00216-019-01630-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01630-7