Abstract
Poly(lactic-co-glycolic acid) particles in the 200–400-nm size range were formulated through nanoprecipitation and solvent evaporation methods. Different concentrations of the polymer and stabilizer (Pluronic® F 68) were tested in order to identify the best conditions for making poly(lactic-co-glycolic acid) particles of suitable size, stable in time, and to be used as carriers for brain-targeting drugs. The particles with the best characteristics for delivery system design were those formulated by nanoprecipitation with an organic/water phase ratio of 2:30, a polymer concentration of 25 mg/mL, and a surfactant concentration of 0.83 mg/mL; their surface charge was reasonably negative (approximately -27 mV) and the average size of the almost monodisperse population was roughly 250 nm. Particle characterization was obtained through ζ-potential measurements, scanning electron microscope observations, and particle size distribution determinations; the latter achieved by both photon-correlation spectroscopy and sedimentation field flow fractionation. Sedimentation field flow fractionation, which is considered more reliable than photon-correlation spectroscopy in describing the possible particle size distribution modifications, was used to investigate the effects of 3 months of storage at 4 °C had on the lyophilized particles.

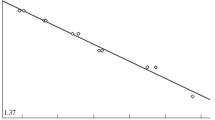
Particle size ditribution from the SdFFF and the PCS techniques





Similar content being viewed by others
References
Lü JM, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q, Chen C (2009) Expert Rev Mol Diagn 9(4):325–341
Armentano I, Dottori M, Fortunati E, Mattioli S, Kenny JM (2010) Polym Degrad Stab 95(11):2126–2146
Bala I, Hariharan S, Ravi Kumar MNV (2004) Ther Drug Carrier Syst 21(5):387–422
Acharya S, Sahoo SK (2011) Adv Drug Deliv Rev 63(3):170–183
Panyam J, Labhasetwar V (2003) Adv Drug Deliv Rev 55:329–347
Fredenberg S, Wahlgren M, Reslow M, Axelsson A (2011) Int J Pharm 415(1–2):34–52
Cruz LJ, Tacken PJ, Fokkink R, Joosten B, Cohen Stuart M, Albericio F, Torensma R, Figdor CG (2011) J Control Release 144(2):118–126
Feczkó T, Tóth J, Dósa Gy, Gyenis J (2011) Chem Eng Process Process Intensif 50(8):846–853
Nafee N, Schneider M, Schaefer UF, Lehr CL (2009) Int J Pharm 381(2):130–139
Astete CE, Sabliov CM (2006) J Biomater Sci Polym Ed 17(3):247–289
Allemann E, Gurny R, Doelker E (1992) Int J Pharm 87:247–253
Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S (1989) Int J Pharm 55:R1–R4
Quintanar-Guerrero D, Ganem-Quintanar A, Allemann E, Fessi H, Doelker E (1998) J Microencapsul 15:107–119
Choi SW, Kwon HY, Kim WS, Kim JH (2002) Colloids Surf A Physicochem Eng Asp 201:283–289
Niwa T, Takeuchi H, Hino T, Kunou N, Kawashima Y (1993) J Control Release 25:89–98
Fessi H, Devissaguet J-P, Puisieux F, Thies C (1992) US Patent 593:522
O'Donnell PB, McGinity JW (1997) Adv Drug Deliv Rev 28(1):25–42
Taluja A, Seok Youn Y, Han Bae Y (2007) Mater Chem 17:4002–4014
Ruozi B, Tosi G, Leo E, Vandelli MA (2007) Talanta 73(1):12–22
Schimpf ME, Caldwell K, Giddings JC (eds) (2000) Field-flow fractionation handbook. Wiley, New York
Kim S, Williams R, Caldwell KD (eds) (2011) Field-flow fractionation in biopolymer analysis. Springer, Heidelberg
Contado C, Argazzi R (2009) J Chromatogr A 1216:9088–9098
Contado C, Argazzi R (2011) J Chromatogr A 1218(27):4179–4187
Williams PS, Giddings JC (1994) Anal Chem 66:4215–4228
Faisant N, Battu S, Senftleber F, Benoit JP, Cardot PJP (2003) J Sep Sci 26:1407–1416
Jeon HJ, Jeong YI, Jang MK, Park YH, Nah JW (2000) Int J Pharm 207:99–108
Mainardes RM, Evangelista RC (2005) Int J Pharm 290:137–144
Santander-Ortega MJ, Jódar-Reyes AB, Csabac N, Bastos-González D, Ortega-Vinuesa JL (2006) J Colloid Interface Sci 302:522–529
Leo E, Scatturin S, Vighi E, Dalpiaz A (2006) J Nanosci Nanotechnol 6(9–10):3070–3079
Csaba N, Camano P, Sanchez A, Domınguez F, Alonso MJ (2005) Biomacromolecules 6:271–278
Abdelwahed W, Degobert G, Stainmesse S, Fessi H (2006) Adv Drug Deliv Rev 58:1688–1713
Contado C, Dalpiaz A, Leo E, Zborowski M, Williams PS (2007) J Chromatogr A 1157:321–335
Jawahar N, Eagappanath T, Venkatesh N, Jubie S, Samanta MK (2009) Int J Pharm Tech Res 1(2):390–393
Song KC, Lee HS, Choung IY, Cho KI, Ahn Y, Choi EJ (2006) Colloids Surf A Physicochem Eng Asp 276:162–167
Holzer M, Vogel V, Mäntele W, Schwartz D, Haase W, Langer K (2009) Eur J Pharm Biopharm 72(2):428–437
Hansen ME, Giddings JC (1989) Anal Chem 61(8):811–819
Lee S, Giddings JC (1988) Anal Chem 60(21):2328–2333
Acknowledgements
This work was financially supported by Unife FAR 2010 and by PRIN2009ZSC5K2_004. Thanks are due to M. Hanuskova for her kind technical support with the PCS analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special issue Analytical Science in Italy with guest editor Aldo Roda.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 639 kb)
Rights and permissions
About this article
Cite this article
Contado, C., Vighi, E., Dalpiaz, A. et al. Influence of secondary preparative parameters and aging effects on PLGA particle size distribution: a sedimentation field flow fractionation investigation. Anal Bioanal Chem 405, 703–711 (2013). https://doi.org/10.1007/s00216-012-6113-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6113-5