Abstract
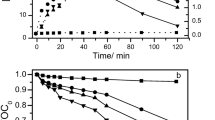
Sulfamethoxazole (SMX) is a synthetic antibiotic widely applied as a bacteriostatic drug to treat a number of diseases. SMX can persist in the environment for long periods of time because of its low biodegradability, which may result in various, direct and indirect, toxicological effects on the environment and on human health. Therefore, we have developed the electrochemical advanced oxidation process (AOP) “electro-Fenton” to degrade SMX in aqueous media. In this work, a detailed study of the evolution of toxicity of SMX and its degradation products in aqueous solutions, during treatment by the electro-Fenton AOP, is described, using the bioluminescence Microtox® method, based on the inhibition of luminescence of marine bacteria Vibrio fischeri. Samples were collected at various electrolysis times and analyzed by HPLC for quantifying the evolution of the degradation products, and their toxicity was measured by the Microtox® method. Our results demonstrated that the toxicity of SMX aqueous solutions varied considerably with the electrolysis time and the applied current intensity. This phenomenon could be explained by the formation and disappearance of several degradation products, including cyclic and/or aromatic intermediates, and short-chain acid carboxylic acids, having a toxicity different of the initial antibiotic. The curves of the % of bacterial luminescence inhibition vs. electrolysis time, corresponding to the evolution of the toxicity of the formed degradation products, were investigated and tentatively interpreted.

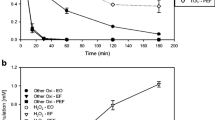
Effect of the applied electrolysis current intensity on the evolution of the V. fischeri bacteria luminescence inhibition with time during the electro-Fenton process of SMX aqueous solutions, after an exposure time of 15 min




Similar content being viewed by others
References
Fenet H, Gomez E, Leclerc M, Casellas C (2006) Environ Risques Santé 5:243–247
Andreozzi R, Marotta R, Praéxus NA (2003) Chemosphere 50:1319–1330
Göbel A, Mc Ardell CS, Joss B, Siegrist H, Giger W (2007) Sci Total Environ 372:361–371
Herber T (2002) Toxicol Lett 131:5–17
Hirsch R, Ternes T, Haberer K, Kratz KL (1999) Sci Total Environ 225:109–118
Boxall AB, Blackwell P, Cavallo R, Kay P, Tolls J (2002) Toxicol Lett 131:19–28
Blackwell PA, Boxall ABA, Kay P, Noble H (2005) J Agric Food Chem 53:2192–2201
Watkinson AJ, Murby EJ, Costanzo SD (2007) Water Res 41:4164–4176
Boxall AB, Fogg LA., Baird DJ, Lewis C, Telfer TC, Kolpin D, Gravell A, (2005) Targeted monitoring study for veterinary medicines in the UK environment. Final report to the UK environmental agency
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Environ Sci Technol 36:1202–1211
Rabiet M, Togola A, Brissaud F, Seidel JL, Budzinski H, Elbaz-Poulichet F (2006) Environ Sci Technol 40:5282–5288
Togola A, Budzinski HJ (2008) J Chromatogr A 1177:150–158
Levi Y (2006) Environ Risques Santé 5:261–265
Ash RJ, Mauck B, Morgan M (2002) US Emerg Infect Dis 8:713–716
Khetan SK, Collins TJ (2007) Chem Rev 107:2319–2364
Henney CR (ed) (1986) A handbook of drugs, 2nd edn. Churchill Livingston, Edinburgh
Sharma VK, Mishra SK, Ray AJ (2006) Chemosphere 62:128–134
Dantas RF, Contreras S, Sans C, Esplugas S (2008) J Hazard Mater 150:790–794
Hu L, Flanders PM, Miller PL, Strathmann TJ (2007) Water Res 41:2612–2626
Beltrán FJ, Aguinaco A, García-Araya JF (2009) Water Res 43:1359–1369
Trovó AG, Nogueira RFP, Agüera A, Sirtori C, Fernandez-Alba AR (2009) Chemosphere 77:1292–1298
González O, Sans C, Esplugas S (2007) J Hazard Mater 146:459–464
Trovó AG, Nogueira RFP, Agüera A, Fernandez-Alba AR, Sirtori C, Malato S (2009) Water Res 43:3922–3931
Li S, Bejan D, McDowell MS, Bunce NJ (2008) J Appl Electrochem 38:151–159
Boudreau J, Bejan D, Li S, Bunce NJ (2010) Ind Eng Chem Res 49:2537–2542
Dirany A, Sirés I, Oturan N, Oturan MA, Chemosphere (2010), in press, doi: 10.1016/j.chemosphere.2010.08.032
Dirany A, Efremova Aaron S, Oturan N, Sirés I, Aaron JJ, Oturan MA (2010) Luminescence 25:232–233
Oturan N, Trajkovska S, Oturan MA, Couderchet M, Aaron JJ (2008) Chemosphere 73:1550–1556
Brillas E, Sirés I, Oturan MA (2009) Chem Rev 109:6570–6631
Escher BI, Bramaz N, Eggen RIL, Richter M (2005) Environ Sci Technol 39:3090–3100
Escher BI, Bramaz N, Maurer M, Richter M, Sutter D, von Kanel C, Zschokke M (2005) Environ Toxicol Chem 24:750–758
Baran W, Sochacka J, Wardas W (2006) Chemosphere 65:1295–1299
Wammer KH, Lapara TM, McNeill K, Arnold WA, Swackhamer DL (2006) Environ Toxicol 25:1480–1486
Santos A, Yustos P, Quintanilla A, García-Ochoa F, Casas JA, Rodríguez JJ (2004) Environ Sci Technol 38:133–138
Kaiser KLE, Palabrica VS (1991) Water Pollut Res J Can 26:361–431
Zazo JA, Casas JA, Molina CB, Quintanilla A, Rodriguez JJ (2007) Environ Sci Technol 41:7164–7170
Acknowledgments
A. Dirany thanks the French government (Ministère de l’Enseignement Supérieur et de la Recherche) for a Ph.D grant. S. Efremova Aaron thanks the University of Paris-Est for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special issue Analytical and Bioanalytical Luminescence with Guest Editor Petr Solich.
Rights and permissions
About this article
Cite this article
Dirany, A., Efremova Aaron, S., Oturan, N. et al. Study of the toxicity of sulfamethoxazole and its degradation products in water by a bioluminescence method during application of the electro-Fenton treatment. Anal Bioanal Chem 400, 353–360 (2011). https://doi.org/10.1007/s00216-010-4441-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-4441-x