Abstract
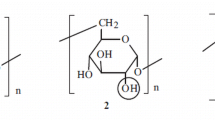
Dextrans from Leuconostoc ssp., α-1,6-linked glucans branched at O-3, were O-methylated in DMSO with lithium dimsyl and methyl iodide under various conditions. Methyl substituent distribution was comprehensively studied in the terminal, internal, and branched glucosyl units and along and over the dextran macromolecules. The order of reactivity was O-2 > O-4 ≥ O-3. The methyl pattern in the glucosyl units significantly deviates from a random distribution with enhanced amounts of un- and trisubstituted moieties. This deviation was found to proceed on macromolecular level by means of ESI-MS of perdeuteromethylated and partially depolymerized methyl dextrans. Heterogeneity was much more pronounced than for methyl amylose prepared under comparable conditions. DS gradients in and over the material are discussed with respect to dextran structure and the mechanism of Li dimsyl alkylation. For comparison, cyanoethyl dextrans were prepared by sodium hydroxide catalyzed addition of acrylonitrile. Monomer analysis of cyanoethyl dextrans revealed that this thermodynamically controlled reaction gave a random substitution pattern with 48% of cyanoethyl groups at O-2, 33% at O-4, and 19% at O-3.

Analysis of the monomer pattern of methyl dextran and comparison with a random model












Similar content being viewed by others
References
Heinze T, Liebert T, Heublein B, Hornig S (2006) Functional polymers based on dextran. Adv Polym Sci 205:199–291
Azzam T, Raskin A, Makovitzki A, Brem H, Vierling P, Lineal M, Domb AJ (2002) Cationic polysaccharides for gene delivery. Macromolecules 35:9947–9953
Chaubet F, Champion J, Maïga O, Mauray S, Jozefonvicz J (1995) Synthesis and structure-anticoagulant property relationships of functionalized dextrans: CMDBS. Carbohydr Polym 28:145–152
Piehler J, Brecht A, Hehl K, Gauglitz G (1999) Protein interactions in covalently attached dextran layers. Colloids Surf B: Biointerfaces 13:325–336
Piehler J, Brecht A, Geckeler KE, Gauglitz G (1996) Surface modification for direct immunoprobes. Biosens Bioelectron 11:579–590
Mann JS, Huang JC, Keana JFW (1992) Molecular amplifiers: synthesis and functionalization of a poly(aminopropyl)dextran bearing a uniquely reactive terminus for univalent attachment to biomolecules. Bioconjugate Chem 3:154–159
Durand A, Marie E, Rotureau E, Leonard M, Dellacherie E (2004) Amphiphilic polysaccharides: useful tools for the preparation of nanoparticles with controlled surface characteristics. Langmuir 20:6956–6963
Rotureau E, Leonard M, Dellacherie E, Durand A (2004) Amphiphilic derivatives of dextran: adsorption at air/water and oil/water interfaces. J Colloid Interface Sci 279:68–77
Aumelas A, Serrero A, Durand A, Dellacherie E, Leonard M (2007) Nanoparticles of hydrophobically modified dextrans as potential drug carrier systems. Colloids Surf B: Biointerfaces 59:74–80
Hornig S, Liebert T, Heinze T (2007) Structure design of multifunctional furoate and pyroglutamate esters of dextran by polymer-analogous reactions. Macromol Bioscience 7:297–306
Liebert T, Hornig S, Hesse S, Heinze T (2005) Nanoparticles on the basis of highly functionalized dextrans. J Am Chem Soc 127:10484–10485
Hornig S, Heinze T, Hesse S, Liebert T (2005) Novel nanoparticles based on dextran esters with unsaturated moieties. Macromol Rapid Commun 26:1908–1912
Hornig S, Heinze T (2007) Nanoscale structures of dextran esters. Carbohydr Polym 68:280–286
Hornig S, Biskup C, Gräfe A, Wotschadlo J, Liebert T, Mohr GJ, Heinze T (2008) Biocompatible fluorescent nanoparticles for pH-sensoring. Soft Matter 4:1169–1172
Seto H, Kawakita H, Ohto K, Harada H, Inoue K (2008) Novel carbonyl-group-containing dextran synthesis by pyranose-2-oxidase and dextransucrase. Carbohydr Res 343:2417–2421
Spurlin HM (1939) Arrangement of substituents in cellulose derivatives. J Am Chem Soc 61:2222–2227
Reuben J (1986) Analysis of the 13C-N.M.R. spectra of hydrolyzed and methanolyze O-methylcelluloses: monomer compositions and models for their description. Carbohydr Res 157:201–213
Mischnick P, Kühn G (1996) Model studies on methyl amyloses: correlation between reaction conditions and primary structure. Carbohydr Res 290:199–207
Liebert T, Heinze T (1998) In: Heinze T, Glasser W (ed) Cellulose derivatives: modification, characterization, and nanostructures. ACS Symposium Series, Washington DC
Mischnick P, Adden R (2008) Fractionation of polysaccharide derivatives and subsequent analysis to differentiate heterogeneities on various hierarchical levels. Macromol Symp 262:1–7
Jozefowicz M, Jozefonvicz J (1997) Randomness and biospecificity: random copolymers are capable of biospecific molecular recognition in living systems. Biomaterials 18:1633–1644
Koschella A, Fenn D, Illy N, Heinze T (2006) Regioselectively functionalized cellulose derivatives: a mini review. Macromol Symp 244:59–73
Steeneken PAM, Woortman AJJ (2008) Surface effects in the acetylation of granular potato starch. Carbohydr Res 343:2278–2284
De Belder AN, Norrman B (1969) The substitution patterns of O-(2-hydroxyethyl)starch and O-(2-hydroxyethyl)dextran. Carbohydr Res 10:391–394
Norrman B (1968) Partial methylation of dextran. Acta Chem Scand 22:1381–1385
De Belder AN, Norrman B (1968) The distribution of substituents in partially acetylated dextran. Carbohydr Res 8:1–6
Ciucanu I, Kerek F (1984) A simple and rapid method for the permethylation of carbohydrates. Carbohydr Res 131:209–217
Sweet DP, Shapiro RH, Albersheim P (1975) Quantitative analysis by various g.l.c. response-factor theories for partially methylated and partially ethylated alditol acetates. Carbohydr Res 40:217–225
Adden R, Müller R, Mischnick P (2006) Analysis of the substituent distribution in the glucosyl units and along the polymer chain of hydroxypropylmethyl celluloses and statistical evaluation. Cellulose 13:459–476
Gonera A, Goclik V, Baum M, Mischnick P (2002) Preparation and structural characterisation of O-aminopropyl starch and amylose. Carbohydr Res 337:2263–2272
Mischnick P, Heinrich J, Gohdes M, Wilke O, Rogmann N (2000) Structure analysis of 1, 4-glucan derivatives. Macromol Chem Phys 201:1985–1995
Arisz PW, Kauw HJJ, Boon JJ (1995) Substituent distribution along the cellulose backbone in O-methylcelluloses using GC and FAB-MS for monomer and oligomer analysis. Carbohydr Res 271:1–14
Larm O, Lindberg B, Svensson S (1971) Studies on the length of the side chains of the dextran elaborated by Leuconostoc mesenteroides NRRL B-512. Carbohydr Res 20:39–48
Wolfrom ML, Thompson A, Timberlake CE (1963) Comparative hydrolysis rates of the reducing disaccharides of d-glucopyranose. Cereal Chem 40:82–86
Basedow AM, Ebert KH, Ederer HJ (1978) Kinetic studies on the acid hydrolysis of dextran. Macromolecules 11:774–781
Bösch A, Nimtz M, Mischnick P (2006) Mechanistic studies on cationic ring-opening polymerisation of cyclodextrin derivatives using various Lewis acids. Cellulose 13:493–507
Adden R, Niedner W, Müller R, Mischnick P (2006) Comprehensive analysis of the substituent distribution in the glucosyl units and along the polymer chain of hydroxyethylmethyl celluloses and statistical evaluation. Anal Chem 78:1146–1157
Adden R, Müller R, Mischnick P (2006) Fractionation of methyl cellulose according to polarity—a tool to differentiate first and second order heterogeneity of the substituent distribution. Macromol Chem Phys 207:954–965
Slodki ME, England RE, Plattner RD, Dick WE (1986) Methylation analyses of NRRL dextrans by capillary gas-liquid chromatography. Carbohydr Res 156:199–206
Khalikova E, Susi P, Korpela T (2005) Microbial dextran-hydrolyzing enzymes: fundamentals and applications. Microbiol Mol Biol Rev 69:306–325
Ioan CE, Aberle T, Burchard W (2000) Structure properties of dextran. 2. Dilute solution. Macromolecules 33:5730–5739
Ioan CE, Aberle T, Burchard W (2001) Light scattering and viscosity behavior of dextran in semidilute solution. Macromolecules 34:326–336
Ioan CE, Aberle T, Burchard W (2001) Structure properties of dextran. 3. Shrinking factors of individual clusters. Macromolecules 34:3765–3771
Rolland-Sabaté A, Mendez-Montealvo MG, Colonna P, Planchot V (2008) Online determination of structural properties and observation of deviations from power law behavior. Biomacromolecules 9:1719–1730
Verraest DL, da Silva LP, Peters JA, van Bekkum H (1996) Synthesis of cyanoethyl inulin, aminopropyl inulin and carboxyethyl inulin. Starch - Stärke 48:191–195
Scanlon JT, Willis DE (1985) Calculation of flame ionization detector relative response factors using the effective carbon number concept. J Chromatogr Sci 23:333–340
Jorgensen AD, Picel KC, Stamoudis VC (1990) Prediction of gas chromatography flame ionization detector response factors from molecular structures. Anal Chem 62:683–689
Acknowledgment
Financial support of A. Vollmer by Konrad-Adenauer Stiftung and of C. Bork by the Federal State of Lower Saxony is gratefully acknowledged. For technical assistance, we thank Silke Lehmann. For helpful discussion, we are very thankful to Prof. Dr. Walther Burchard, University of Freiburg, Germany.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
216_2009_3013_MOESM1_ESM.pdf
An extended version of the experimental part, more examples of cyanoethyldextrans (5, Fig. 2) as well as additional calculations on monomer methyl distribution (c 0–c 4, x i ) is available as electronic supplementary material (PDF 87 kb)
Rights and permissions
About this article
Cite this article
Vollmer, A., Voiges, K., Bork, C. et al. Comprehensive analysis of the substitution pattern in dextran ethers with respect to the reaction conditions. Anal Bioanal Chem 395, 1749–1768 (2009). https://doi.org/10.1007/s00216-009-3013-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-3013-4