Abstract
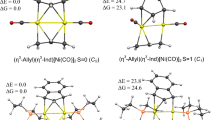
Quantum chemical investigations at the BP86/def2-SVP, BP86/def2-TZVPP and BP86/TZ2P+ levels of theory have been done for the series of AlH2+ complexes that carry carbodiphosphorane and analogues called tetrylones [X(PPh3)2–AlH2]+ (Al-XPPh) (X = C–Pb) using charge and partitioning methods. The most stable structures of Al-XPPh have been found for carbone CPPh as a mildly side-on style in carbone complex Al-CPPh, while the heavier tetrylone adducts Al-SiPPh−Al-PbPPh have significantly different side-on fashions SiPPh−PbPPh, which exhibit the more acute bending angles in tilted forms linked to AlH2+ fragment. Bond dissociation energies (BDEs), De (kcal/mol), slightly decrease from the strongest bonded carbone, Al-CPPh, to the weaker bonded heavier homologues. The bulky tetrylone ligands XPPh have significantly influenced to the Al-X bond strength in complexes Al-XPPh when calculating BDEs with dispersion interaction. The NBO analysis revealed that the [X(PPh3)2 → AlH2]+ donation comes mainly from the σ- and π-contributions of the ligands. The EDA–NOCV calculations showed that the bond sturdiness of the Al–X bond results from the decrease in [X(PPh3)2 → AlH2]+ donation and electrostatic attraction. The EDA–NOCV data also indicated that AlH2+–tetrylone complexes exhibit not only (PPh3)2X → AlH2+ strong σ-donors and weak π-donors but also (PPh3)2X ← AlH2+ weak π-back donation as π–π electrons shared in complexes.









Similar content being viewed by others
References
Petz W, Öxler F, Neumüller B, Tonner R, Frenking G (2009) Carbodiphosphorane C(PPh3)2 as a single and twofold lewis base with boranes: synthesis, crystal structures and theoretical studies on [H3B{C(PPh3)2}] and [{(μ-H)H4B2}{C(PPh3)2}]+. Eur J Inorg Chem 2009:4507–4517
Petz W, Frenking G (2010) Carbodiphosphoranes and related ligands. Top Organomet Chem 30:49–92
Tonner R, Frenking G (2008) Divalent carbon(0) chemistry, part 1: parent compounds. Chem Eur J 14:3260–3272
Tonner R, Frenking G (2008) Divalent carbon(0) chemistry, part 2: protonation and complexes with main group and transition metal lewis acids. Chem Eur J 14:3273–3289
Frenking G, Tonner R (2009) Divalent carbon(0) compounds. Pure Appl Chem 81:597–614
Ramirez F, Desai NB, Hansen B, McKelvie N (1961) Hexaphenylcarbodiphosphorane, (C6H5)3PCP(C6H5)3. J Am Chem Soc 83:3539–3540
Hardy GE, Zink JI, Kaska WC, Baldwin JC (1978) Structure of triboluminescence of polymorphs of hexaphenylcarbodiphosphorane. J Am Chem Soc 100:8001–8002
Klein S, Tonner R, Frenking G (2010) Carbodicarbenes and related divalent carbon(0) compounds. Chem Eur J 16:10160–10170
Takagi N, Shimizu T, Frenking G (2009) Divalent E(0) Compounds (E = Si–Sn). Chem Eur J 15:8593–8604
Takagi N, Frenking G (2011) Divalent Pb(0) compounds. Theoret Chem Acc 129:615–623
Frenking G, Hermann M, Andrada DM, Holzmann N (2016) Donor–acceptor bonding in novel low-coordinated compounds of boron and Group-14 atoms C–Sn. Chem Soc Rev 45:1129–1144
Frenking G, Tonner R, Klein S, Takagi N, Shimizu T, Krapp A, Pandeyc KK, Parameswaran P (2014) New bonding modes of carbon and heavier Group 14 atoms Si–Pb. Chem Soc Rev 43:5106–5139
Takagi N, Shimizu T, Frenking G (2009) Divalent silicon(0) compounds. Chem Eur J 15:3448–3456
Zhao L, Hermann M, Holzmann N, Frenking G (2017) Dative bonding in main group compounds. Coord Chem Rev 344:163–204
Mondal KC, Roesky HW, Schwarzer MC, Frenking G, Niepötter B, Wolf H, Herbst-Irmer R, Stalke D (2013) A stable singlet biradicaloid siladicarbene: (L:)2Si. Angew Chem Int Ed 52:2963–2967
Xiong Y, Yao S, Tan G, Inoue S, Driess M (2013) A cyclic germadicarbene (“Germylone”) from germyliumylidene. J Am Chem Soc 135:5004–5007
Xiong Y, Yao S, Inoue S, Epping JD, Driess M (2013) A cyclic silylone (“Siladicarbene”) with an electron-rich silicon(0) atom. Angew Chem Int Ed 52:7147–7150
Celik MA, Frenking G, Neumüller B, Petz W (2013) Exploiting the twofold donor ability of carbodiphosphoranes: theoretical studies of [(PPh3)2C → EH2]q(Eq = Be, B+, C2+, N3+, O4+) and synthesis of the dication [(Ph3P)2C = CH2]2+. Chem Plus Chem 78:1024–1032
Petz W, Neumüller B, Klein S, Frenking G (2011) Syntheses and crystal structures of [Hg{C(PPh3)2}2][Hg2I6] and [Cu{C(PPh3)2}2]I and comparative theoretical study of carbene complexes [M(NHC)2] with carbone complexes [M{C(PH3)2}2] (M = Cu+, Ag+, Au+, Zn2+, Cd2+, Hg2+). Organometallics 30:3330–3339
Vicente J, Singhal AR, Jones PG (2002) New ylide−, alkynyl−, and mixed alkynyl/ylide − gold(I) complexes. Organometallics 21:5887–5900
Reitsamer C, Schuh W, Kopacka H, Wurst K, Peringer P (2009) Synthesis and structure of the first heterodinuclear PCP − pincer − CDP complex with a Pd − Au d8 − d10 pseudo-closed-shell interaction. Organometallics 28:6617–6620
Reitsamer C, Schuh W, Kopacka H, Wurst K, Ellmerer E, Peringer P (2011) The first carbodiphosphorane complex with two palladium centers attached to the CDP carbon: assembly of a single-stranded di-Pd helicate by the PCP pincer ligand C(dppm)2. Organometallics 30:4220–4223
Petz W, Dehnicke K, Holzmann N, Frenking G, Neumüller B (2011) The reaction of BeCl2 with carbodiphosphorane C(PPh3)2: experimental and theoretical studies. Z Anorg Allg Chem 637:1702–1710
Petz W, Weller F, Uddin J, Frenking G (1999) Reaction of carbodiphosphorane Ph3PCPPh3 with Ni(CO)4. experimental and theoretical study of the structures and properties of (CO)3NiC(PPh3)2 and (CO)2NiC(PPh3)2. Organometallics 18:619–626
Sundermeyer J, Weber K, Peters K, von Schnering HG (1994) Modeling surface reactivity of metal oxides: synthesis and structure of an ionic organorhenyl perrhenate formed by ligand-induced dissociation of covalent Re2O7. Organometallics 13:2560–2562
Kinjo R, Donnadieu B, Celik MA, Frenking G, Bertrand G (2011) Synthesis and characterization of a neutral tricoordinate organoboron isoelectronic with amines. Science 333:610–613
Celik MA, Sure R, Klein S, Kinjo R, Bertrand G, Frenking G (2012) Borylene complexes (BH)L2 and nitrogen cation complexes (N+)L2: isoelectronic homologues of carbones CL2. Chem Eur J 18:5676–5692
Inés B, Patil M, Carreras J, Goddard R, Thiel W, Alcarazo M (2011) Synthesis, structure, and reactivity of a dihydrido borenium cation. Angew Chem Int Ed 50:8400–8403
Nguyen TAN, Frenking G (2012) Transition-metal complexes of tetrylones [(CO)5W–E(PPh3)2] and tetrylenes [(CO)5W–NHE] (E = C–Pb): a theoretical study. Chem Eur J 18:12733–12748
Nguyen TAN, Frenking G (2013) Structure and bonding of tetrylone complexes [(CO)4W{E(PPh3)2}] (E = C–Pb). Molec Phys 111:2640–2646
Nguyen TAN, Tran DS, Huynh TPL, Le TH, Duong TQ, Nguyen TT, Vo TC, Van TP, Dang TH (2017) Can tetrylone act in a similar fashion to tetrylene in Ni(CO)2 complexes? a theoretical study based on a comparison using DFT calculations. Z Anorg Allg Chem 643:826–838
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al (2009) Gaussian 09. Gaussian Inc., Wallingford, CT
Ahlrichs R, Bär M, Häser M, Horn H, Kölmel C (1989) Electronic structure calculations on workstation computers: the program system turbomole. Chem Phys Lett 162:165–169
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Perdew JP (1986) Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys Rev B 33:8822–8824
Schäfer A, Horn H, Ahlrichs R (1992) Fully optimized contracted Gaussian basis sets for atoms Li to Kr. Chem Phys 97:2571–2577
Metz B, Stoll H, Dolg M (2000) Small-core multiconfiguration-Dirac–Hartree–Fock-adjusted pseudopotentials for post-d main group elements: application to PbH and PbO. J Chem Phys 113:2563–2569
Andrae D, Häußermann U, Dolg M, Stoll H, Preuß H (1990) Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Theor Chim Acta 77:123–141
Weigend F (2005) Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305
Neese F (2003) An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix. J Comput Chem 24:1740–1747
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comput Chem 32:1456–1465
Goerigk L, Grimme S (2011) A thorough benchmark of density functional methods for general main group thermochemistry, kinetics, and noncovalent interactions. Phys Chem Chem Phys 13:6670–6688
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–746
Morokuma K (1971) Molecular orbital studies of hydrogen bonds. III. C=O···H–O hydrogen bond in H2CO···H2O and H2CO···2H2O. J Chem Phys 55:1236–1244
Ziegler T, Rauk A (1977) On the calculation of bonding energies by the Hartree Fock Slater method. Theor Chim Acta 46:1–10
Mitoraj MP, Michalak A, Ziegler T (2009) A combined charge and energy decomposition scheme for bond analysis. J Chem Theory Comput 5:962–975
Mitoraj M, Michalak A (2007) Natural orbitals for chemical valence as descriptors of chemical bonding in transition metal complexes. J Mol Model 13:347–355
Mitoraj M, Michalak A (2007) Donor–acceptor properties of ligands from the natural orbitals for chemical valence. Organometallics 26:6576–6580
Gte Velde, Bickelhaupt FM, Baerends EJ, Guerra CF, van Gisbergen SJA, Snijders JG, Ziegler T (2001) Chemistry with ADF. J Comput Chem 22:931–967
Snijders JG, Vernooijs P, Baerends EJ (1981) Roothaan–Hartree–Fock–Slater atomic wave functions: single-zeta, double-zeta, and extended Slater-type basis sets for 87Fr–103Lr. At Data Nucl Data Tables 26:483–509
Krijn J, Baerends EJ (1984) Fit functions in the HFS-method. Internal Report, Vrije Universiteit Amsterdam, Amsterdam
Evan Lenthe, Ehlers A, Baerends EJ (1999) Geometry optimizations in the zero order regular approximation for relativistic effects. J Chem Phys 110:8943–8953
Kaska WC, Mitchell DK, Reichelderfer RF (1973) Transition metal complexes of hexaphenylcarbodiphosphorane. J Organomet Chem 47:391–402
Kaska WC, Mitchell DK, Reichelderfer RF, Korte WD (1974) Interaction of phosphorus ylides with transition metal carbonyl compounds. Triphenylphosphinemethylene and bis(triphenylphosphine)carbon. Comparative chemistry. J Am Chem Soc 96:2847–2854
Acknowledgements
Nguyen Thi Ai Nhung thanks Prof. Dr. Gernot Frenking for allowing the continuous use of her own resources within Frenking’s group. The programs used in the studies were run via the Erwin/Annemarie clusters operated by Reuti (Thomas Reuter) at the Philipps-Universität Marburg, Germany. This research is funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under Grant No. 104.06-2017.303 (Nguyen Thi Ai Nhung).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nhung, N.T.A., Loan, H.T.P., Son, P.T. et al. Theoretical assessment of donor–acceptor complexes [X(PPh3)2 → AlH2]+ (X = C–Pb): structures and bonding. Theor Chem Acc 138, 67 (2019). https://doi.org/10.1007/s00214-019-2456-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-019-2456-8