Abstract
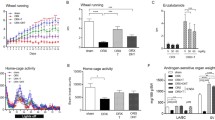
There is increasing evidence that the biological activity of myostatin (MSTN), a negative regulator of muscle growth, is affected by training but also anabolic steroids. In this study, we analyzed the effects of the frequently abused anabolic steroid methandienone (Md) on the hypothalamic–pituitary–testicular axis and androgen-sensitive tissues in intact rats performing a treadmill training to simulate the situation of abusing athletes. The anabolic effects were correlated with the expression of members of the MSTN signaling cascade. Md treatment resulted in a significant stimulation of anabolic activity of the levator ani muscle, which was further increased by training, while prostate and seminal vesicle weights decreased in conformance with hormone concentrations of LH and testosterone. In gastrocnemius muscle, mRNA expression of genes of the MSTN signaling cascade (MSTN, Smad7 and MyoD) was reduced by training but not after Md treatment, in soleus muscle MSTN and its inhibitors, follistatin (FLST) and Smad-7 were only affected after training in combination with Md treatment. In summary, our data demonstrate that Md treatment of intact rats results in anabolic effects which are enhanced in combination with physical activity. Interestingly, the anabolic activity on the levator ani was increased in combination with training, although the levator ani muscle was not specifically stimulated by our training protocol. In the m. gastrocnemius and soleus, the anabolic effects correlate with changes in the expression patterns of genes involved in MSTN signaling. Our data provide evidence that the decrease in the weight of androgen-sensitive sexual glands, observed after Md treatment, is caused by a suppression of endogenous testosterone synthesis. These observations provide new insights into the molecular mechanisms of the interaction between anabolic steroids, training and MSTN signaling during skeletal muscle adaptation.





Similar content being viewed by others
Abbreviations
- AAS:
-
Anabolic-androgenic steroids
- ACTIIB:
-
Activin type IIB receptor
- AR:
-
Androgen receptor
- Bw:
-
Body weight
- FLST:
-
Follistatin
- Md:
-
Methandienone
- MSTN:
-
Myostatin
- s. c.:
-
Subcutaneous
- TGF-ß:
-
Transforming growth factor-β family of proteins
References
Aoki MS, Soares AG, Miyabara EH, Baptista IL, Moriscot AS (2009) Expression of genes related to myostatin signaling during rat skeletal muscle longitudinal growth. Muscle Nerve 40(6):992–999
Beiglbock W, Brummund W (1960) On the problem of the anabolic effect of testosterone derivatives. A report on experiences with dianabol. Med Welt 22:1192–1205 [Article in German]
Boada LD, Zumbado M, Torres S, Lopez A, Diaz-Chico BN, Cabrera JJ, Luzardo OP (1999) Evaluation of acute and chronic hepatotoxic effects exerted by anabolic-androgenic steroid stanozolol in adult male rats. Arch Toxicol 73:465–472
Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS (2002) Functional improvement of dystrophic muscle by myostatin blockade. Nature 420:418–421
Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS (2005) Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J 19(6):543–549
Chen Y, Lebrun JJ, Vale W (1996) Regulation of transforming growth factor beta- and activin-induced transcription by mammalian Mad proteins. Proc Natl Acad Sci USA 93:12992–12997
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem 162(1):156–159
de Souza RWA, Gonçalves W, Cavalcante WLG, Pai-Silva MD, Gallacci M (2010) Nandrolone stimulates MyoD expression during muscle regeneration in the condition of myonecrosis induced by Bothrops jararacussu venom poisoning. J Toxicol Environ Health A 73:934–943
Desaulles PA, Kraehenbuehl C, Schuler W, Bein HJ (1959) Experimental study of dianabol, a new anabolic agent. 17 alpha-Methyl-17 beta-hydroxy-androstane-1, 4-diene-3-one. Schweiz Med Wochenschr 89:1313–1318
Diel P, Friedel A, Geyer H, Kamber M, Laudenbach-Leschowsky U, Schänzer W, Thevis M, Vollmer G, Zierau O (2007) Characterisation of the pharmacological profile of desoxymethyltestosterone (Madol), a steroid misused for doping. Toxicol Lett 169(1):64–71
Diel P, Baadners D, Schlüpmann K, Velders M, Schwarz JP (2008) C2C12 myoblastoma cell differentiation and proliferation is stimulated by androgens and associated with a modulation of myostatin and Pax7 expression. J Mol Endocrinol 40:231–241
Diel P, Schiffer T, Geisler S, Hertrampf T, Mosler S, Schulz S, Wintgens KF, Adler M (2010) Analysis of the effects of androgens and training on myostatin propeptide and follistatin concentrations in blood and skeletal muscle using highly sensitive Immuno PCR. Mol Cell Endocrinol 330(1–2):1–9
Eriksson A, Kadi F, Malm C, Thornell LE (2005) Skeletal muscle morphology in power-lifters with and without anabolic steroids. Histochem Cell Biol 124(2):167–175
Feraudi M, Weicker H (1985) Effects of training and methandrostenolone (an anabolic steroid) on energy metabolism in the guinea pig: changes in enzyme activities in gastrocnemius muscle and myocardium. Int J Biochem 17(11):1191–1205
Forbes D, Jackman M, Bishop A, Thomas M, Kambadur R, Sharma M (2006) Myostatin auto-regulates its expression by feedback loop through Smad7 dependent mechanism. J Cell Physiol 206:264–272
Foss GL (1960) Some experiences with a new anabolic steroid (methandrostenolone). Br Med J 1(5182):1300–1305
Friedel A (2006) Molekulare Mechanismen der Skelettmuskeladaptation. Dissertation, German Sport University, Cologne
Friedel A, Geyer H, Kamber M, Laudenbach-Leschowsky U, Schänzer W, Thevis M, Vollmer G, Zierau O, Diel P (2006) Tetrahydrogestrinone is a potent but unselective binding steroid and affects glucocorticoid signalling in the liver. Toxicol Lett 164(1):16–23
Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Ménissier F, Massabanda J, Fries R, Hanset R, Georges M (1997) A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet 17(1):71–74
Jensky NE, Sims JK, Dieli-Conwright CM, Sattler FR, Rice JC, Schroeder ET (2010) Exercise does not influence myostatin and follistatin messenger RNA expression in young women. J Strength Cond Res 24(2):522–530
Joulia-Ekaza D, Cabello G (2006) Myostatin regulation of muscle development: molecular basis, natural mutations, physiopathological aspects. Exp Cell Res 312:2401–2414
Kim JS, Cross JM, Bamman MM (2005) Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab 288(6):E1110–E1119
Kollias HD, McDermott JC (2008) Transforming growth factor-beta and myostatin signaling in skeletal muscle. J Appl Physiol 104:579–587
Legerlotz K, Smith HK (2008) Role of MyoD in denervated, disused and exercised muscle. Muscle Nerve 38:1087–1100
Llewelynn W (2009) Dianabol (methandrostenolone, methandienone). In: Llewelynn W (ed) Anabolics, 9th edn. Jupiter, Florida, pp 207–211
Louis E, Raue U, Yang Y, Jemiolo B, Trappe S (2007) Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol 103(5):1744–1751
Ma K, Mallidis C, Artaza J, Taylor W, Gonzales-Cadavid N, Bhasin S (2001) Characterization of 5′-regulatory region of human myostatin gene: regulation by dexamethasone in vitro. Am J Physiol Endocrinol Metab 281(6):1128–1136
Matsakas A (2004) Effect of exercise on the mRNA expression of growth factors, metabolic genes and myosin heavy chain isoforms in skeletal muscles of the rat. Dissertation, German Sport University, Cologne
Matsakas A, Friedel A, Hertrampf T, Diel P (2005) Short-term endurance training results in a muscle-specific decrease of myostatin mRNA content in the rat. Acta Physiol Scand 183:299–307
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387(6628):83–90
Parr MK, Flenker U, Schänzer W (2010) Sports-related issues and biochemistry of natural and synthetic anabolic substances. Endocrinol Metab Clin North Am 39(1):45–57
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):2002–2007
Roselli CE (1998) The effect of anabolic-androgenic steroids on aromatase activity and androgen receptor binding in the rat preoptic area. Brain Res 792(2):271–276
Roth SM, Martel GF, Ferrell RE, Metter EJ, Hurley BF, Rogers MA (2003) Myostatin gene expression is reduced in humans with heavy-resistance strength training: a brief communication. Exp Biol Med (Maywood) 228(6):706–709
Sarkar M, Dutta Borah BK, Bandopadhayay S, Meyer HH, Prakash BS (2009) Season of the year influences semen output and concentrations of testosterone in circulation of yaks (Poephagus grunniens L.). Anim Reprod Sci 115(1–4):300–305
Schuelke M, Wagner KR, Stolz LE, Hübner C, Riebel T, Kömen W, Braun T, Tobin JF, Lee SJ (2004) Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350(26):2682–2688
Singh R, Bhasin S, Braga M, Artaza JN, Pervin S, Taylor WE, Krishnan V, Sinha SK, Rajavashisth TB, Jasuja R (2009) Regulation of myogenic differentiation by androgens: cross talk between androgen receptor/ß-catenin and follistatin/transforming growth factor-ß signaling pathways. Endocrinology 150:1259–1268
Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, Storer TW, Casaburi R, Shen R, Bhasin S (2002) Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab 283(1):E154–E164
Steele RE, Didato F, Steinetz BG (1977) Relative importance of 5alpha reduction for the androgenic and LH-inhibiting activities of delta-4-3-ketosteroids. Steroids 29(3):331–348
Takahashi M, Tatsugi Y, Kohno T (2004) Endocrinological and pathological effects of anabolic-androgenic steroid in male rats. Endocr J 51(4):425–434
Wheeler GD, Singh M, Pierce WD, Epling WF, Cumming DC (1991) Endurance training decreases serum testosterone levels in men without change in luteinizing hormone pulsatile release. J Clin Endocrinol Metab 72(2):422–425
Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, Hill JJ, Jalenak M, Kelley P, Knight A, Maylor R, O'Hara D, Pearson A, Quazi A, Ryerson S, Tan XY, Tomkinson KN, Veldman GM, Widom A, Wright JF, Wudyka S, Zhao L, Wolfman NM (2003) Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun 300:965–967
Willoughby DS (2004) Effects of heavy resistance training on myostatin mRNA and protein expression. Med Sci Sports Exerc 36(4):574–582
Wynn V, Landon J (1961) A study of the androgenic and some related effects of methandienone. Br Med J 1:998–1003
Zhao H, Shi W, Chen H, Warburton D (2000) Smad7 and Smad6 differentially modulate transforming growth factor beta induced inhibition of embryonic lung morphogenesis. J Biol Chem 275:23992–23997
Zoth N, Weigt C, Laudenbach-Leschowski U, Diel P (2010) Physical activity and estrogen treatment reduce visceral body fat and serum levels of leptin in an additive manner in a diet induced animal model of obesity. J Steroid Biochem Mol Biol 122(1–3):100–105
Acknowledgments
We want to thank the World Anti Doping Agency (WADA) for supporting this study and Bayer Schering for analysis of serum LH and FSH concentrations. Furthermore, we want to thank Mrs. Felicitas Graf for general assistance in statistical analysis.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mosler, S., Pankratz, C., Seyfried, A. et al. The anabolic steroid methandienone targets the hypothalamic–pituitary–testicular axis and myostatin signaling in a rat training model. Arch Toxicol 86, 109–119 (2012). https://doi.org/10.1007/s00204-011-0740-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-011-0740-z