Abstract
Summary
The aim of this systematic review and meta-analysis is to study the utility of the commonly used bone turnover markers in evaluating disease activity in patients with Paget’s disease of bone before and after treatment with bisphosphonates. We found good correlation between the bone turnover marker concentrations and disease activity assessed by bone scintigraphy.
Introduction
Paget’s disease of bone is a common skeletal disorder of the elderly. Bone turnover marker concentrations are used for diagnosis and follow-up. We aimed to compare the available bone turnover markers and determine their utility in assessing disease activity when compared to quantitative bone scintigraphy.
Methods
We conducted a systematic review and meta-analysis searching MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Scopus. We evaluated total alkaline phosphatase (total ALP), bone-specific alkaline phosphatase (bone ALP), procollagen type 1 amino-terminal propeptide (P1NP), serum, and urine C-terminal telopeptide (uCTx and sCTx, respectively), and urine N-terminal telopeptide (uNTx). The main outcome of interest was the correlation of disease activity with concentrations of bone turnover markers in Paget’s disease patients before and after treatment with bisphosphonates. Correlation coefficients were pooled across studies using the random effects model.
Results
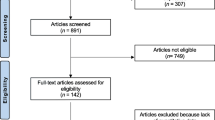
We included 17 observational studies and one trial reporting on 953 patients. Prior to treatment, all studied bone turnover markers had moderate to strong correlation with scintigraphic indices (correlation coefficients ranging from 0.58 to 0.80) with no statistically significant difference between the bone turnover markers overall (p = 0.08). P1NP, uNTx, and bone ALP tend to have higher correlation with scintigraphy. After starting treatment with bisphosphonate, there was moderate to strong correlation with disease activity with all markers except bone ALP (correlation coefficients ranging from 0.43 to 0.70).
Conclusion
The findings of this meta-analysis suggest the Paget’s disease activity is best monitored by following P1NP levels. However, total ALP, bone ALP, and uNTx are good alternatives as markers of disease activity in untreated patients. Total ALP and uNTx can be useful in following patients with Paget’s disease after treatment if P1NP is not available. Clinicians, however, should take availability, cost, and the presence of liver disease into consideration when deciding which bone turnover marker is most appropriate when evaluating patients with Paget’s disease.




Similar content being viewed by others
References
Corral-Gudino L, Borao-Cengotita-Bengoa M, Del Pino-Montes J, Ralston S (2013) Epidemiology of Paget’s disease of bone: a systematic review and meta-analysis of secular changes. Bone Meta-Anal Rev 55(2):347–352
Wermers RA, Tiegs RD, Atkinson EJ, Achenbach SJ, Melton LJ 3rd (2008) Morbidity and mortality associated with Paget’s disease of bone: a population-based study. J Bone Miner Res. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. 23(6):819–25
Altman RD, Bloch DA, Hochberg MC, Murphy WA (2000) Prevalence of pelvic Paget’s disease of bone in the United States. J Bone Miner Res. [Research Support, Non-U.S. Gov’t]. 15(3):461–5
Siris ES, Ottman R, Flaster E, Kelsey JL (1991) Familial aggregation of Paget’s disease of bone. J Bone Miner Res. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. 6(5):495–500
Merlotti D, Gennari L, Galli B, Martini G, Calabro A, De Paola V et al (2005) Characteristics and familial aggregation of Paget’s disease of bone in Italy. J Bone Miner Res 20(8):1356–1364
Laurin N, Brown JP, Morissette J, Raymond V (2002) Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am J Hum Genet. [Research Support, Non-U.S. Gov’t]. 70(6):1582–8
Hocking LJ, Lucas GJ, Daroszewska A, Mangion J, Olavesen M, Cundy T (2002) et al. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Hum Mol Genet. [Research Support, Non-U.S. Gov’t]. 11(22):2735–9
Alvarez L, RicOs C, Peris P, GuaNabens N, Monegal A, Pons F, et al (2000) Components of biological variation of biochemical markers of bone turnover in Paget’s bone disease. Bone. [Comparative Study]. 26(6):571–6
Bonnin MR, Moragues C, Nolla JM, Liron FJ, Roig-Escofet D, Navarro MA (1998) Evaluation of circulating type I procollagen propeptides in patients with Paget’s disease of bone. Clin Chem Lab Med. [Comparative Study]. 36(1):53–5
Alexandersen P, Peris P, Guanabens N, Byrjalsen I, Alvarez L, Solberg H, et al (2005) Non-isomerized C-telopeptide fragments are highly sensitive markers for monitoring disease activity and treatment efficacy in Paget’s disease of bone. J Bone Miner Res. [Evaluation Studies]. 20(4):588–95
Alvarez L, Guanabens N, Peris P, Vidal S, Ros I, Monegal A, et al (2001) Usefulness of biochemical markers of bone turnover in assessing response to the treatment of Paget’s disease. Bone. [Clinical Trial]. 29(5):447–52
Roodman GD, Windle JJ (2005) Paget disease of bone. J Clin Invest. [Research Support, U.S. Gov’t, P.H.S. Review]. 115(2):200–8
Reid IR, Miller P, Lyles K, Fraser W, Brown JP, Saidi Y et al (2005) Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. N Engl J Med 353(9):898–908
Reid IR, Miller P, Lyles K, Fraser W, Brown JP, Saidi Y et al (2005) Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. New Engl J Med 353(9):898–908
Leung KS, Fung KP, Sher AH, Li CK, Lee KM (1993) Plasma bone-specific alkaline phosphatase as an indicator of osteoblastic activity. J Bone Joint Surg Br. [Comparative Study]. 75(2):288–92
Shankar S, Hosking DJ (2006) Biochemical assessment of Paget’s disease of bone. J Bone Miner Res 21:P22–P27
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. [Guideline Research Support, Non-U.S. Gov’t]. 151(4):264–9, W64
Wells G SB, O’connell D, Peterson J, Welch V, Losos M, Tugwell P (2014) The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed June, 2014 [cited 2014 May, 2014]
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. [Research Support, Non-U.S. Gov’t]. 343:d5928
David Machine MJC, Stephen J (2007) Walters medical statistics — a text book for the health sciences, 4th edn
Alvarez L, Peris P, Pons F, Guanabens N, Herranz R, Monegal A et al (1997) Relationship between biochemical markers of bone turnover and bone scintigraphic indices in assessment of Paget’s disease activity. Arthritis Rheum 40(3):461–468
Pons F, Alvarez L, Peris P, Guanabens N, Vidal-Sicart S, Monegal A et al (1999) Quantitative evaluation of bone scintigraphy in the assessment of Paget’s disease activity. Nucl Med Commun 20(6):525–528
Meunier PJ, Salson C, Mathieu L, Chapuy MC, Delmas P, Alexandre C, et al (1987) Skeletal distribution and biochemical parameters of Paget’s disease. Clin Orthop Relat Res. [Comparative Study]. (217):37–44
Woitge HW, Pecherstorfer M, Li Y, Keck AV, Horn E, Ziegler R, et al (1999) Novel serum markers of bone resorption: clinical assessment and comparison with established urinary indices. J Bone Miner Res. [Comparative Study]. 14(5):792–801
Bachiller-Corral J, Diaz-Miguel C, Morales-Piga A (2013) Monostotic Paget’s disease of the femur: a diagnostic challenge and an overlooked risk. Bone 57(2):517–521
Woitge HW, Oberwittler H, Heichel S, Grauer A, Ziegler R, Seibel MJ (2000) Short- and long-term effects of ibandronate treatment on bone turnover in Paget disease of bone. Clin Chem 46(5):684–690
de la Piedra C, Rapado A, Diaz Diego EM, Diaz Martin MA, Aguirre C, Lopez Gavilanes E, et al (1996) Variable efficacy of bone remodeling biochemical markers in the management of patients with Paget’s disease of bone treated with tiludronate. Calcif Tissue Int. [Comparative Study Research Support, Non-U.S. Gov’t]. 59(2):95–9
Ulivieri FM, Piodi LP, Marotta G, Marchelli D, Corradini C, Parravicini L et al (2006) Usefulness of osteoprotegerin in assessing responses to neridronate treatment in Paget’s disease of bone. J Orthop Traumatol 7(4):192–194
Reid IR, Davidson JS, Wattie D, Wu F, Lucas J, Gamble GD et al (2004) Comparative responses of bone turnover markers to bisphosphonate therapy in Paget’s disease of bone. Bone 35(1):224–230
Randall AG, Kent GN, Garcia-Webb P, Bhagat CI, Pearce DJ, Gutteridge DH et al (1996) Comparison of biochemical markers of bone turnover in Paget disease treated with pamidronate and a proposed model for the relationships between measurements of the different forms of pyridinoline cross-links. J Bone Miner Res 11(8):1176–1184
Alvarez L, Peris P, Guanabens N, Vidal S, Quinto L, Monegal A et al (2004) Long-term biochemical response after bisphosphonate therapy in Paget’s disease of bone. Proposed intervals for monitoring treatment. Rheumatology (Oxford) 43(7):869–874
Garnero P, Gineyts E, Schaffer AV, Seaman J, Delmas PD (1998) Measurement of urinary excretion of nonisomerized and beta-isomerized forms of type I collagen breakdown products to monitor the effects of the bisphosphonate zoledronate in Paget’s disease. Arthritis Rheum. [Clinical Trial Randomized Controlled Trial]. 41(2):354–60
Griffith K, Pearson D, Parker C, Thorpe S, Vincent RM, Hosking DJ (2001) The use of a whole body index with bone scintigraphy to monitor the response to therapy in Paget’s disease. Nucl Med Commun 22(10):1069–1075
Zati A, Colori BC, Bonfiglioli Stagni S, Mignani A (2011) Pain in Paget’s disease: a retrospective study of treatment efficacy. Neuro Endocrinol Lett 32(2):127–132
Garnero P, Gineyts E, Schaffer AV, Seaman J, Delmas PD (1998) Measurement of urinary excretion of nonisomerized and beta-isomerized forms of type I collagen breakdown products to monitor the effects of the bisphosphonate zoledronate in Paget’s disease. Arthritis Rheum 41(2):354–360
Alexandersen P, Peris P, Guanabens N, Byrjalsen I, Alvarez L, Solberg H et al (2005) Non-isomerized C-telopeptide fragments are highly sensitive markers for monitoring disease activity and treatment efficacy in Paget’s disease of bone. J Bone Miner Res 20(4):588–595
Ulivieri FM, Marchelli D, Como G, Valente G, Messa P, Raimondi AR, et al (2006) Increased osteoprotegerin in Italian haemodialysis patients. Osteoporos Int. [Comment Letter]. 17(12):1822–3; author reply 4
Ralston SH (2013) Clinical practice. Paget’s disease of bone. N Engl J Med. [Review]. 368(7):644–50
Selby PL, Davie MW, Ralston SH, Stone MD (2002) Guidelines on the management of Paget’s disease of bone. Bone. [Guideline Research Support, Non-U.S. Gov’t]. 31(3):366–73
Ingram RT, Collazo-Clavell M, Tiegs R, Fitzpatrick LA (1996) Paget’s disease is associated with changes in the immunohistochemical distribution of noncollagenous matrix proteins in bone. J Clin Endocrinol Metab 81(5):1810–1820
Shankar S, Hosking DJ (2006) Biochemical assessment of Paget’s disease of bone. J Bone Mineral Res: Of J Am Soc Bone Mineral Res 21(Suppl 2):P22–P27
Delmas PD (1999) Biochemical markers of bone turnover in Paget’s disease of bone. J Bone Miner Res Off J Am Soc Bone Miner Res 14(Suppl 2):66–69
Acknowledgments
This study was supported by a contract from the Endocrine Society.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Appendix 1
Appendix 1
Search strategy
Ovid
Database(s)
EMBASE 1988 to 2012 Week 42, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) 1946 to Present, EBM Reviews - Cochrane Central Register of Controlled Trials October 2012, EBM Reviews - Cochrane Database of Systematic Reviews 2005 to September 2012
Scopus
-
1
TITLE-ABS-KEY(“Osteitis Deformans” or (paget W/4 bone) or (pagets W/4 bone) or “ostitis deformans”)
-
2
TITLE-ABS-KEY(“biological marker*” or biomarker* or “serum marker*” or “laboratory marker*” or “immunologicmarker*” or (surrogate W/1 endpoint*) or (surrogate W/1 “end point*”) or “biologic marker*” or “immune marker*” or “clinical marker*” or “biochemical marker*” or “biological indicator*” or bioindicator* or “Alkaline Phosphatase”or “alcalic phosphatase” or “alkali phosphatase” or “alkalic phosphatase” or “alkaline monophosphoesterase” or “alkaline phosphohydrolase” or “alkaline phosphomonoesterase” or “alkalinic phosphatase” or “basic phosphatase” or “orthophosphoric monoester phosphohydrolase” or procollagen or “collagen precursor” or “proto-collagen” or protocollagen or precollagen or “collagen type 1” or “collagen 1” or “collagen i” or “type 1 collagen” or vitrogen or “type I collagen” or P1NP or P1NP or telopeptide or creatinine or creatinin or kreatinine or methylglycocyamimine or “1 methylglycocyamidine” or “1 methylhydantoin 1 imide” or “2 imino 1 methyl 4imidazolinone”)
-
3
TITLE-ABS-KEY(serum or blood or urine or urinary)
-
4
TITLE-ABS-KEY( (evidence W/1 based) OR (meta W/1 analys*) OR (systematic* W/2 review*) OR guideline OR (control* W/2 stud*) OR (control* W/2 trial*) OR (randomized W/2 stud*) OR (randomized W/2 trial*) or random* or “latin square” or crossover or “cross-over” or placebo* or (doubl* N5 blind*) or (doubl* N5 mask*) or (singl* N5 blind*) or (singl* N5 mask*) or (tripl* N5 blind*) or (tripl* N5 mask*) or (trebl* N5 blind*) or (trebl* N5 mask*) or “comparative study” OR “comparative survey” OR “comparative analysis” OR “cohort study” OR “cohort survey” OR “cohort analysis” OR “longitudinal study” OR “longitudinal survey” OR “longitudinal analysis” OR “retrospective study” OR “retrospective survey” or “retrospective analysis” OR “prospective study” OR “prospective survey” OR “prospective analysis” OR “population study” OR “population survey” OR “population analysis” OR “concurrent study” OR “concurrent survey” OR “concurrent analysis” or “incidence study” OR “incidence survey” OR “incidence analysis” OR “follow-up study” OR “follow-up survey” OR “follow-up analysis” or “observational study” OR “observational survey” OR “observational analysis” OR “case study” OR “case series” OR “clinical series” OR “case studies” or “clinical study” OR “clinical trial” or “evaluation study” OR “evaluation survey” OR “evaluation analysis” or “twin study” OR “twin survey” OR “twin analysis” or “validation study” OR “validation survey” OR “validation analysis” or “experimental study” OR “experimental analysis” or “field study” OR “field survey” OR “field analysis” or “in vivo study” OR “in vivo analysis” or “panel study” OR “panel survey” OR “panel analysis” or “pilot study” OR “pilot survey” OR “pilot analysis” or “prevention study” OR “prevention survey” OR “prevention analysis” or “replication study” OR “replication analysis” or “theoretical study” OR “theoretical analysis” or “trend study” OR “trend survey” OR “trend analysis” or “multivariate analysis”)
-
5
1 and 2 and 3 and 4
-
6
PMID (0*) OR PMID (1*) OR PMID (2*) OR PMID (3*) OR PMID (4*) OR PMID (5*) OR PMID (6*) OR PMID (7*) OR PMID (8*) OR PMID (9*)
-
7
5 and not 6
-
8
DOCTYPE(le) OR DOCTYPE(ed) OR DOCTYPE(bk) OR DOCTYPE(er) OR DOCTYPE(no) OR DOCTYPE(sh)
-
9
7 and not 8
Rights and permissions
About this article
Cite this article
Al Nofal, A.A., Altayar, O., BenKhadra, K. et al. Bone turnover markers in Paget’s disease of the bone: A Systematic review and meta-analysis. Osteoporos Int 26, 1875–1891 (2015). https://doi.org/10.1007/s00198-015-3095-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3095-0