Abstract
Introduction and hypothesis
An animal model of vaginal distention (VD) was developed to reproduce the acute urethral injury and deficiency underlying stress urinary incontinence (SUI). Data on the chronic effects of urethral trauma and the recovery process are still scarce. We investigated acute, short- and long-term histomorphological and molecular changes in the urethra of rats post 12-h intermittent VD.
Methods
We evaluated the urethra of four groups of female rats (n = 72): control without trauma, 1 h, 7 days and 30 days post VD. We compared the gene and protein expression of the VEGF and NGF growth factors, collagens (COL1a1 and COL3a1), desmin, smooth muscle myosin (MYH11), skeletal muscle myosins (MYH1, MYH2 and MYH3) and cell proliferation marker MKi67. We used real-time RT-qPCR, and immunohistochemistry.
Results
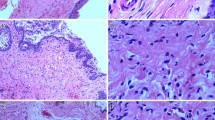
Histology showed urethral damage after VD mainly involving the muscular layers. VEGF, NGF, desmin and MKi67 mRNA were significantly upregulated in the urethras of rats 1-h post VD compared with controls (P < 0.05 for all). By 7 days post trauma, COL1a1, MYH11 and MYH3 genes were overexpressed compared with controls (p < 0.05 for all). The COL3a1 protein level was increased by 2.6 times by day 7, while MYH2 protein was significantly decreased (around two times) from 7 to 30 days post VD compared with controls (p < 0.05 for both).
Conclusions
The 12-h intermittent VD causes chronic alterations in the urethra represented by increased COL3a1 and decreased MYH2 protein levels in the long term. The model can potentially be used to study the mechanisms of urethral injury and recovery as well as the physiopathology of SUI.







Similar content being viewed by others
References
Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4–20.
Rud T, Andersson KE, Asmussen M, et al. Factors maintaining the intraurethral pressure in women. Investig Urol. 1980;17(4):343–7.
Cannon TW, Wojcik EM, Ferguson CL, et al. Effects of vaginal distension on urethral anatomy and function. BJU Int. 2002;90:403–7.
Phull HS, Pan HQ, Butler RS, et al. Vulnerability of continence structures to injury by simulated childbirth. Am J Physiol Renal Physiol. 2011;301(3):F641–9.
Huang J, Cheng M, Ding Y, et al. Modified vaginal dilation rat model for postpartum stress urinary incontinence. J Obstet Gynaecol Res. 2013;39(1):256–63.
Damaser MS, Broxton-King C, Ferguson C, et al. Functional and neuroanatomical effects of vaginal distension and pudendal nerve crush in the female rat. J Urol. 2003;170:1027–31.
Pan HQ, Kerns JM, Lin DL, et al. Dual simulated childbirth injury delays anatomic recovery. Am J Physiol Renal Physiol. 2009;296:F277–83.
Prantil RL, Jankowski RJ, Kaiho Y, et al. Ex vivo biomechanical properties of the female urethra in a rat model of birth trauma. Am J Physiol Renal Physiol. 2007;292:F1229–37.
Hong SH, Piao S, Kim IG, et al. Comparison of three types of stress urinary incontinence rat models: electrocauterization, pudendal denervation and vaginal distension. Urology. 2013;81:465.e1–6.
Rocha MA, Sartori MGF, Simões MJ, et al. The impact of pregnancy and childbirth in the urethra of female rats. Int Urogynecol J. 2007;18:645–51.
Pan HQ, Kerns JM, Lin DL, et al. Increased duration of simulated childbirth injuries results in increased time to recovery. Am J Physiol Regul Integr Comp Physiol. 2007;292(4):R1738–44.
Hofer MD, Cheng EY, Bury MI, et al. Analysis of primary urethral wound healing in the rat. Urology. 2014;84(1):246.e1–7.
Sengupta P. The laboratory rat: relating its age with Human’s. Int J Prev Med. 2013;4(6):624–30.
Jiang HH, Damaser MS. Animal models of stress urinary incontinence. Handb Exp Pharmacol. 2011;202:45–67.
Phillips JI, Davies I. The comparative morphology of the bladder and urethra in young and old female C57BL/Icrfat mice. Exp Geront. 1980;15:551–62.
Woo LL, Hijaz A, Kuang M, et al. Over expression of stem cell homing cytokines in urogenital organs following vaginal distention. J Urol. 2007;177:1568–72.
Lenis AT, Kuang M, Woo LL, et al. Impact of parturition on chemokine homing factor expression in the vaginal distention model of stress urinary incontinence. J Urol. 2013;189:1588–94.
Birot OJG, Koulmann N, Peinnequin A, et al. Exercise-induced expression of vascular endothelial growth factor mRNA in rat skeletal muscle is dependent on fibre type. J Physiol. 2003;552(1):213–21.
Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–81.
Gerdes J, Schwab U, Lemke H, et al. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31(1):13–20.
Wood HM, Kuang M, Woo L, et al. Cytokine expression after vaginal distention of different durations in virgin Sprague-Dawley rats. J Urol. 2008;180:753–9.
Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am. 2002;84-A(5):822–32.
Bornemann A, Schmalbruch H. Desmin and vimentin in regenerating muscles. Muscle Nerve. 1992;15:14–20.
Beamish JA, He P, Kottke-Marchant K, et al. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev. 2010;16:467–91.
Schiaffino S, Rossi AC, Smerdu V, et al. Developmental myosins: expression patterns and functional significance. Skelet Muscle. 2015;5:22.
Birk DE, Fitch JM, Babiarz JP, et al. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990;95:649–57.
Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210.
Acknowledgements
For excellent assistance, the authors are grateful to Eloísa D. Castro, Gabriel A. Alves and Graciele A. Oliveira.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Financial support
This research was supported by Fundação de Apoio à Pesquisa do Estado de São Paulo FAPESP (no. 20.254/2011).
Rights and permissions
About this article
Cite this article
Bortolini, M.A.T., Feitosa, S.M., Bilhar, A.P.M. et al. Molecular and histomorphological evaluation of female rats’ urethral tissues after an innovative trauma model of prolonged vaginal distention: immediate, short-term and long-term effects. Int Urogynecol J 30, 465–476 (2019). https://doi.org/10.1007/s00192-018-3634-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-018-3634-2