Abstract
Introduction and hypothesis
An intravaginal device (Uresta) is currently available for the treatment of stress urinary incontinence (SUI). Case-series data on its effectiveness exist; however, controlled data are lacking. The objective of this study is to determine the short-term efficacy of the Uresta device using a randomized placebo controlled trial. The hypothesis is that the Uresta device might significantly reduce urinary loss.
Methods
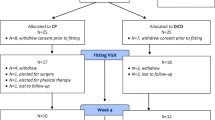
A single blind randomized controlled trial was conducted among women with urodynamic SUI recruited from a single urogynecology unit. Participants were randomized to receive the Uresta device or a placebo vaginal silastic ring placed high in the vagina for the duration of a pad test. Pad tests were performed before and after device placement. The primary outcome was the achievement of a 50 % or greater reduction in pad weight after device placement, in a comparison of the two groups. Sample size calculation showed a need for 18 subjects per group. Fisher’s exact test was used to analyze the primary outcome. Research Ethics Board approval was obtained.
Results
Eighteen subjects per group completed the study protocol. The percentage of patients who achieved the primary outcome was 66.7 % in the Uresta group and 22.2 % in the placebo group (p = 0.01). The baseline demographic data were similar in the two groups. There were no adverse events during the test period.
Conclusions
The Uresta intravaginal continence device significantly reduces the short-term objective measures of urine loss due to SUI. Further study to assess subjective outcomes and long-term patient satisfaction is required.
Similar content being viewed by others
References
Minassian VA, Drutz HP, Al-Badr A. Urinary incontinence as a worldwide problem. Int Urogyn J. 2003;82:327–38.
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–6.
Shaikh S, Ong EK, Glavind K, Cook J, N’Dow JMO. Mechanical devices for urinary incontinence in women. Cochrane Database Syst. 2006. Rev 7:CD001756
Veirhout ME, Lose G. Preventative vaginal and intraurethral devices in the treatment of female urinary stress incontinence. Curr Opin Obstet Gynecol. 1997;9:325–8.
Farrell SA, Baydock S, Amir B, Fanning C. Effectiveness of a new self-positioning pessary for the management of urinary incontinence in women. Am J Obstet Gynecol. 2007;196:474.e1–474.e4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by an internal grant from the hospital’s Department of Obstetrics and Gynecology.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Lovatsis, D., Best, C. & Diamond, P. Short-term Uresta efficacy (SURE) study: a randomized controlled trial of the Uresta continence device. Int Urogynecol J 28, 147–150 (2017). https://doi.org/10.1007/s00192-016-3090-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-016-3090-9