Abstract
Introduction and hypothesis
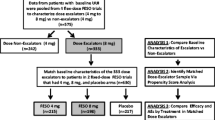
This study evaluated the efficacy and safety of flexible-dose fesoterodine and factors associated with dose escalation in subjects with overactive bladder (OAB).
Methods
In this 12-week, open-label study, 331 adults with OAB symptoms for ≥3 months, ≥8 micturitions and ≥3 urgency episodes per 24 h and who reported at least “some moderate” bladder-related problems were treated with fesoterodine 4 mg once daily for 4 weeks, with the option to escalate to 8 mg for the remaining 8 weeks based on discussion of efficacy and tolerability with the investigator. Factors influencing dose escalation were identified using stepwise logistic regression. Efficacy was assessed via 3-day bladder diaries and patient-reported outcomes.
Results
Of the subjects, 59 % dose escalated at week 4; 93 % of escalators cited insufficient clinical response. The decision to escalate was most often made by the subject (alone or with the investigator). Improvements from baseline were observed in diary and patient-reported outcomes at weeks 4 and 12. Smaller improvements in micturition frequency and worse bladder-related problems at week 4 were significantly associated with increased likelihood of dose escalation; baseline micturition frequency, age, sex, body mass index, antimuscarinic-associated adverse events and OAB symptom duration were not. Non-escalators had greater improvement from baseline to week 4 than escalators; by week 12, improvement was similar among escalators and non-escalators. Fesoterodine was well tolerated.
Conclusions
Treatment with flexible-dose fesoterodine improved bladder diary and patient-reported outcomes. Lower clinical response was related to dose escalation; after escalation, response in escalators approached that of non-escalators.


Similar content being viewed by others
Abbreviations
- AE:
-
Adverse event
- BMI:
-
Body mass index
- BSW:
-
Benefit, Satisfaction and Willingness to Continue
- CI:
-
Confidence interval
- ICIQ-SF:
-
International Consultation on Incontinence Questionnaire–Short Form
- OAB:
-
Overactive bladder
- PPBC:
-
Patient Perception of Bladder Condition
- SAE:
-
Serious adverse event
- SAGA:
-
Self-Assessment of Goal Achievement
- SD:
-
Standard deviation
- TSQ:
-
Treatment Satisfaction Questionnaire
- UPS:
-
Urgency Perception Scale
- UUI:
-
Urgency urinary incontinence
References
Milsom I, Irwin DE (2007) A cross-sectional, population-based, multinational study of the prevalence of overactive bladder and lower urinary tract symptoms: results from the EPIC study. Eur Urol Suppl 6:4–9
Reeves P, Irwin D, Kelleher C, Milsom I, Kopp Z, Calvert N et al (2006) The current and future burden and cost of overactive bladder in five European countries. Eur Urol 50:1050–1057
Andersson KE, Chapple CR, Cardozo L, Cruz F, Hashim H, Michel MC et al (2009) Pharmacological treatment of overactive bladder: report from the International Consultation on Incontinence. Curr Opin Urol 19:380–394
Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D (2008) The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol 54:543–562
Novara G, Galfano A, Secco S, D’Elia C, Cavalleri S, Ficarra V et al (2008) A systematic review and meta-analysis of randomized controlled trials with antimuscarinic drugs for overactive bladder. Eur Urol 54:740–763
Benner JS, Nichol MB, Rovner ES, Jumadilova Z, Alvir J, Hussein M et al (2010) Patient-reported reasons for discontinuing overactive bladder medication. BJU Int 105:1276–1282
Carr LK (2008) Overactive bladder. Can J Urol 15(Suppl 1):32–36; discussion 36
Michel MC, Staskin D (2011) Understanding dose titration: overactive bladder treatment with fesoterodine as an example. Eur Urol Suppl 10:8–13
Chapple CR, Rosenberg MT, Brenes FJ (2009) Listening to the patient: a flexible approach to the use of antimuscarinic agents in overactive bladder syndrome. BJU Int 104:960–967
Chapple C, Van Kerrebroeck P, Tubaro A, Haag-Molkenteller C, Forst HT, Massow U et al (2007) Clinical efficacy, safety, and tolerability of once-daily fesoterodine in subjects with overactive bladder. Eur Urol 52:1204–1212
Nitti VW, Dmochowski R, Sand PK, Forst HT, Haag-Molkenteller C, Massow U et al (2007) Efficacy, safety and tolerability of fesoterodine for overactive bladder syndrome. J Urol 178:2488–2494
Khullar V, Rovner ES, Dmochowski R, Nitti V, Wang J, Guan Z (2008) Fesoterodine dose response in subjects with overactive bladder syndrome. Urology 71:839–843
Wyndaele JJ, Goldfischer ER, Morrow JD, Gong J, Tseng LJ, Guan Z et al (2009) Effects of flexible-dose fesoterodine on overactive bladder symptoms and treatment satisfaction: an open-label study. Int J Clin Pract 63:560–567
Dmochowski RR, Peters KM, Morrow JD, Guan Z, Gong J, Sun F et al (2010) Randomized, double-blind, placebo-controlled trial of flexible-dose fesoterodine in subjects with overactive bladder. Urology 75:62–68
Coyne KS, Margolis MK, Hsieh R, Vats V, Chapple CR (2011) Validation of the urinary sensation scale (USS). Neurourol Urodyn 30:360–365
Coyne KS, Matza LS, Kopp Z, Abrams P (2006) The validation of the Patient Perception of Bladder Condition (PPBC): a single-item global measure for patients with overactive bladder. Eur Urol 49:1079–1086
Cardozo L, Coyne KS, Versi E (2005) Validation of the Urgency Perception Scale. BJU Int 95:591–596
Coyne K, Revicki D, Hunt T, Corey R, Stewart W, Bentkover J et al (2002) Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res 11:563–574
Kelleher CJ, Cardozo LD, Khullar V, Salvatore S (1997) A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol 104:1374–1379
Reese PR, Pleil AM, Okano GJ, Kelleher CJ (2003) Multinational study of reliability and validity of the King’s Health Questionnaire in patients with overactive bladder. Qual Life Res 12:427–442
Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P (2004) ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 23:322–330
Pleil AM, Coyne KS, Reese PR, Jumadilova Z, Rovner ES, Kelleher CJ (2005) The validation of patient-rated global assessments of treatment benefit, satisfaction, and willingness to continue—the BSW. Value Health 8:S25-S34
Piault E, Evans CJ, Espindle D, Kopp Z, Brubaker L, Abrams P (2008) Development and validation of the Overactive Bladder Satisfaction (OAB-S) Questionnaire. Neurourol Urodyn 27:179–190
Brubaker L, Khullar V, Piault E, Evans CJ, Bavendam T, Beach J et al (2011) Goal attainment scaling in patients with lower urinary tract symptoms: development and pilot testing of the Self-Assessment Goal Achievement (SAGA) questionnaire. Int Urogynecol J 22:937–946
Wyndaele JJ, Goldfischer ER, Morrow JD, Gong J, Tseng LJ, Choo MS (2011) Patient-optimized doses of fesoterodine improve bladder symptoms in an open-label, flexible-dose study. BJU Int 107:603–611
Staskin D, Khullar V, Michel MC, Morrow JD, Sun F, Guan Z et al (2011) Effects of voluntary dose escalation in a placebo-controlled, flexible-dose trial of fesoterodine in subjects with overactive bladder. Neurourol Urodyn 30:1480–1485
Acknowledgments
This study was sponsored by Pfizer Ltd. Medical writing assistance was provided by Patricia B. Leinen, Ph.D., and Colin P. Mitchell, Ph.D., from Complete Healthcare Communications, Inc. and was funded by Pfizer Ltd.
Conflicts of interest
LC received funding during the preceding year for research, lecturing, or consultancies from Astellas and Pfizer and has done research consultancy or advisory work for Astellas, Pfizer, Ethicon, TEVA, Merck, and Eli Lilly. TH, JR, and AW have received research monies, honoraria, or consultancy fees from Pfizer, Astellas, Watson Pharma, and Orion Pharma. CEB is an employee of Pfizer France. AD is an employee of Pfizer Ltd. IK was an employee of Pfizer Ltd at the time this study was conducted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cardozo, L., Hall, T., Ryan, J. et al. Safety and efficacy of flexible-dose fesoterodine in British subjects with overactive bladder: insights into factors associated with dose escalation. Int Urogynecol J 23, 1581–1590 (2012). https://doi.org/10.1007/s00192-012-1804-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-012-1804-1