Abstract
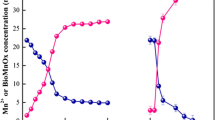
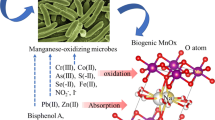
This work studied the effect of biogenic manganese oxides (Bio-MnOx) on carbofuran degradation.The results showed that 21.05 % and 90.63 % carbofuran, respectively, were degraded in 4 days by Bio-MnOx with and without NaN3 at initial pH 4.80, whereas carbofuran was hardly degraded by chemical manganese oxides in the same condition. Bio-MnOx promoted carbofuran hydrolysis by changing the pH of the environment and encouraged carbofuran phenol cleavage by its oxidization. Both the oxidation of carbofuran phenol by Bio-MnOx and the reoxidation of the released Mn(II) by Mn(II)-oxidizing microorganisms ensured the continuous reactivity of Bio-MnOx and prevented the secondary pollution of Mn(II). Carbofuran phenol was the major transformation product in the degradation and was further oxidized into small organic molecules as monitored by a GC/MS analyzer. This report offers an efficient, feasible, and no-secondary-pollution approach to controlling carbofuran pollution.






Similar content being viewed by others
References
Bachman J, Patterson HH (1999) Photodecomposition of the carbamate pesticide carbofuran: kinetics and the influence of dissolved organic matter. Environ Sci Technol 33:874–881
Caldas SS, Demoliner A, Costa FP, D’Oca MGM, Primel EG (2010) Pesticide residue determination in groundwater using solid-phase extraction and high-performance liquid chromatography with diode array detector and liquid chromatography-tandem mass spectrometry. J Braz Chem Soc 21:642–650
Chapman R, Cole C (1982) Observations on the influence of water and soil pH on the persistence of insecticides. J Environ Sci Heal B 17:487–504
Chin-Pampillo JS, Ruiz-Hidalgo K, Masis-Mora M, Carazo-Rojas E, Rodriguez-Rodriguez CE (2015) Adaptation of biomixtures for carbofuran degradation in on-farm biopurification systems in tropical regions. Environ Sci Pollut R 22:9839–9848
Dong D, Nelson YM, Lion LW, Shuler ML, Ghiorse WC (2000) Adsorption of Pb and Cd onto metal oxides and organic material in natural surface coatings as determined by selective extractions: new evidence for the importance of Mn and Fe oxides. Water Res 34:427–436
Feng X, Ou L-T, Ogram A (1997) Plasmid-mediated mineralization of carbofuran by Sphingomonas sp. strain CF06. Appl Environ Microb 63:1332–1337
Forrez I, Carballa M, Verbeken K, Vanhaecke L, Ternes T, Boon N, Verstraete W (2010) Diclofenac oxidation by biogenic manganese oxides. Environ Sci Technol 44:3449–3454
Liao S, Zhou J, Wang H, Chen X, Wang H, Wang G (2013) Arsenite oxidation using biogenic manganese oxides produced by a deep-sea manganese-oxidizing bacterium, Marinobacter sp. MnI7-9. Geomicrobiol J 30:150–159
Lower BH, Hochella MF, Lower SK (2005) Putative mineral-specific proteins synthesized by a metal reducing bacterium. Am J Sci 305:687–710
Ma YS, Kumar M, Lin JG (2009) Degradation of carbofuran-contaminated water by the Fenton process. J Environ Sci Heal A 44:914–920
Mahalakshmi A, Arabindoo B, Palanichamy A, Murugesan V (2007) Photocatalytic degradation of carbofuran using semiconductor oxides. J Hazard Mater 143:240–245
Nguyen TPO et al (2014) Genetic and metabolic analysis of the carbofuran catabolic pathway in Novosphingobium sp KN65.2. Appl Microbiol Biot 98:8235–8252
Plese LPD, Paraiba LC, Foloni LL, Trevizan LRP (2005) Kinetics of carbosulfan hydrolysis to carbofuran and the subsequent degradation of this last compound in irrigated rice fields. Chemosphere 60:149–156
Sabirova JS, Cloetens L, Vanhaecke L, Forrez I, Verstraete W, Boon N (2008) Manganese-oxidizing bacteria mediate the degradation of 17α-ethinylestradiol. Microb Biotechnol 1:507–512
Saputra E, Muhammad S, Sun H, Ang H-M, Tadé MO, Wang S (2013) Manganese oxides at different oxidation states for heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions. Appl Catal B 142:729–735
Tariq MI, Afzal S, Hussain I (2004) Pesticides in shallow groundwater of Bahawalnagar, Muzafargarh, D.G Khan and Rajan Pur districts of Punjab, Pakistan. Environ Int 30:471–479
Tu JJ, Yang ZD, Hu C, Qu JH (2014) Characterization and reactivity of biogenic manganese oxides for ciprofloxacin oxidation. J Environ Sci-China 26:1154–1161. doi:10.1016/S1001-0742(13)60505-7
Villalobos M, Lanson B, Manceau A, Toner B, Sposito G (2006) Structural model for the biogenic Mn oxide produced by Pseudomonas putida. Am Mineral 91:489–502
Acknowledgments
This project was supported by the Fundamental Research Funds for the Central Universities (Grant No. 2013PY122).
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhiqiang Liu and Jia Wang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, Z., Wang, J., Qian, S. et al. Carbofuran Degradation by Biogenic Manganese Oxides. Bull Environ Contam Toxicol 98, 420–425 (2017). https://doi.org/10.1007/s00128-016-1940-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1940-2