Abstract
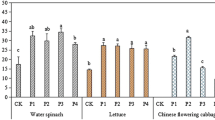
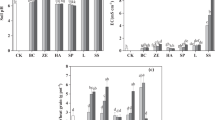
The use of conventional methods to clean up the soil is very expensive and destructive to the ecosystem. The concept of phytoextraction has been introduced to safely manage soils contaminated with heavy metals. However, using plants to remediate polluted soils is a lengthy process. This has necessitated the use of amendments to potentially enhance solubilization of metals in order to increase their bioavailability in the soil solution. A pot experiment was conducted to study the effect of some selected pH lowering amendments [elemental sulphur and (NH4)2SO4] on the solubility and availability of Cd and Zn. The application of these amendments resulted in a decrease in the pH of the soil. The decrease in pH significantly enhanced the solubilization and the mobility of Cd and Zn into the soil solution. The CaCl2 extraction protocol was employed to study the effects of the various amendments on the mobility of Cd and Zn.



Similar content being viewed by others
References
Allison LE (1965) Methods of soil analysis, part 2. Chemical and microbiological properties. In: Black CA (ed) Agronomy monograph 9, 2nd edn. ASA, Madison, pp 1367–1378
Blaylock MJ, Huang JW (2000) Phytoextraction of metals. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals—using plants to clean-up the environment. Wiley, New York, pp 53–70
Brown SL, Chaney RL, Angle JS, Baker AJM (1994) Zinc and cadmium uptake of Thlaspi caerulescens grown in nutrient solution. Soil Sci Soc Am J 59(125):133
Cui Y, Dong Y, Li H, Wang Q (2004) Effect of elemental sulphur on solubility of soil heavy metals and their uptake by maize. Environ Int J 30:323–328
Kayser A, Wenger K, Gupta SK, Furverts G, Schulin R (2002) Comparison of NTA and elemental sulphur as potential soil amendments in phytoremediation. Soil Sediment Contam 11(5):655–672
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London, pp 313–404
Martinez CE, Motto HL (2000) Solubility of lead and copper added to mineral soils. Environ Pollut 107:153–158
Meers E, Ruttens A, Hopgood M, Lesage E, Tack FMG (2005a) Potential of Brassic rapa, Cannabis sativa, Helianthus annuus and Zea mays for phytoextraction of heavy metals from calcareous dredged sediment derived soils. Chemosphere 61:561–572
Meers E, Ruttens A, Hopgood MJ, Samson D, Tack FMG (2005b) Comparison of EDTA and EDDS as potential soil amendments for enhanced phytoextraction of heavy metals. Chemosphere 58:1011–1022
Menon M, Sandra H, Madeleine S, Günthardt G, Rainer S (2007) Effect of heavy metal soil pollution and acid rain on growth and water use efficiency of a young model forest ecosystem. Plant Soil 297:171–183
Navarro MC, Perez-Sirvent C, Martinez-Sanchec MJ, Vida J, Tovar PJ, Bech J (2008) Abandoned mine sites as a source of contamination by heavy metals: a case study in a semi-arid zone. J Geochem Explor 86:183–193
Nishi M, Joginder S, Sachendra B, Afshan Q, Anil V (2007) Arbuscular mycorrhizal fungi: a potential tool for phytoremediation. J Plant Sci 2(127):140
Peck HD Jr (1975) Biological oxidation and reduction of inorganic compounds of sulfur. http://web.anl.gov/PCS/acsfuel/preprint%20archive/Files/202PHILADELPHIA04-750076.pdf. Accessed 20 Jun 2014
Prasad MNV (2006) Bioremediation: an innovative technology to decontaminate the environment through biodiversity and bio-geo-chemical cycling. Science Pub Inc, Bremen, Germany
Pueyo M, Lopez- Sanchez JF, Rauret G (2004) Assessment of CaCl2, NaNO3 and (NH)NO3 extraction procedures for the study of Cd, Cu, Pb and Zn extractability in contaminated soils. Anal Chim Acta 504:217–226
Raymond AW, Felix EO (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol 2011:1–20
Reddy KJ, Wang L, Gloss SP (1995) Copper, zinc and lead in acidic environments. Plant Soil 171:53–58
Van Nevel L, Merten SJ, Oorts K, Verheyen K (2007) Phytoextraction of metals from soils: how far from practice? Environ Pollut 150:34–40
Van Ranst E (2006) Properties and management of soils in the tropics. Chapter 2, 31–32
Van Ranst E, Verloo M, Demeyer A, Pauwels JM (1999) Manual for the soil chemistry and fertility laboratory—analytical methods for soils and plants, Equipment and management of consumables. NUGI 835, Ghent, Belgium, 243 pp
Vassilev A, Van Gronsveld J, Yordanov I (2002) Cadmium phytoextraction: present state, biological backgrounds and research needs. Bulg J Plant Physiol 28:68–95
Wang AD, Angle JS, Chaney RL, Delorme TA, Reeves RD (2006) Soil pH effects on uptake of Cd and Zn by Thlaspi caerulescens. Plant Soil 281:325–337. doi:10.1007/s11104-005-4642-9
Zuangh P, Zou B, Li NY, Li ZA (2009) Heavy metal contamination in soils and food crops around Dabaoshan mine in Guangdong, China: implication for human health. Environ Geochem Health 31(707):715
Acknowledgments
This research was conducted at the Department of Analytical and Applied Chemistry and Echo Chemistry, Ghent University, Belgium.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amoakwah, E., Van Slycken, S. & Essumang, D.K. Comparison of the Solubilizing Efficiencies of Some pH Lowering (Sulphur and (NH4)2SO4) Amendments on Cd and Zn Mobility in Soils. Bull Environ Contam Toxicol 93, 187–191 (2014). https://doi.org/10.1007/s00128-014-1319-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1319-1