Abstract
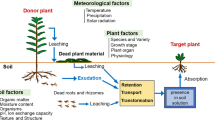
Allelopathy is an important mechanism of plant interference mediated by the release of plant-produced secondary metabolites or decomposition products of microbes to the aerial or soil environment. It plays a key role in natural as well as cultivated ecosystems. Allelochemicals are released into the soil rhizosphere by a variety of mechanisms, including volatilization, decomposition of residues, and root exudation. Along with inhibitory/stimulatory effects of allelochemicals, several other ecological roles of these chemicals, including plant defense, nutrient chelation, and regulation of soil biota, have been reported. Wheat is extensively studied and used as an allelopathic crop, and numerous chemicals are reported to be released from the wheat living plants and decomposing residues. In this review, we presented a contemporary synthesis of the existing data that how wheat allelopathy can be exploited: (a) to biologically control the insects, pests, and diseases, (b) to enhance the soil quality by adding nutrients for crop plants during decomposition from residues and ameliorate soil environment for microbes, (c) to increase the crop diversification by rotation while reducing the weeds and pests infestation, (d) to develop the low-cost biological pesticides with a novel mode of action from crop plants, and (e) to confer tolerance against abiotic stresses. Based on our hypothetical concepts and previous evidences, we briefly discussed the mode of action of allelochemicals, and extent and rate of their production based on crop growth stage. We also addressed the interaction of root exudates and allelochemicals with soil biotic and abiotic components to explore the role of allelopathy in rhizosphere ecology.


Similar content being viewed by others
References
Abbas RN, Tanveer A, Khaliq A, Iqbal A, Ghaffari AR, Matloob A, Maqsood Q (2013) Maize (Zea mays L.) germination, growth and yield response to foliar application of Moringa oleifera Lam. leaf extracts. Crop Environ 4:39–45
Aiyaz M, Divakara ST, Chandranayaka S, Niranjana SR (2015) Efficacy of seed hydropriming with phytoextracts on plant growth promotion and antifungal activity in maize. Int J Pest Manag 2:153–160
Akemo MC, Regnier EE, Bennett MA (2000) Weed suppression in spring-sown rye (Secale cereale)-pea (Pisum sativum) cover crop mixes. Weed Technol 14:545–549
Aliferis KA, Jabaji S (2011) Metabolomics—a robust bioanalytical approach for the discovery of the modes-of-action of pesticides: a review. Pestic Biochem Phys 100:105–117
Alsaadawi IS (2001) Allelopathic influence of decomposing wheat residues in agroecosystem. J Crop Prod 4:185–196
Alsaadawi IS (2008) Allelopathic influence of decomposing wheat residues in agroecosystem. J Crop Prod 4:185–196
Amb MK, Ahluwalia AS (2016) Allelopathy: potential role to achieve new milestones in rice. Rice Sci 23:165–183
Ambika SR (2012) Multifaceted attributes of allelochemicals and mechanism of allelopathy. In: Cheema ZA, Farooq M, Wahid A (eds) Allelopathy: current trends and future applications. Springer, Germany, pp 113–143
An M, Johnson IR, Lovett JV (1993a) Mathematical modeling of allelopathy: biological response to allelochemicals and its interpretation. J Chem Ecol 19:2379–2388
An M, Johnson IR, Lovett JV (1993b) Mathematical modeling of allelopathy: biological response to allelochemicals and its interpretation. J Chem Ecol 19:2379–2388
An M, Pratley J, Haig T (1998) Allelopathy: from Concept to Reality. In: Michalk DL, Pratley JE (eds) Proceeding of the 9th Australian Agronomy Conference. Wagga Wagga, Australia, pp 563–566
Anaya AL (1999) Allelopathy as a tool in the management of biotic resources in agroecosystems. Crit Rev Plant Sci 18:697–739
Anaya AL, Waller GR, Owuor PO, Friedman J, Chou CH, Suzuki T, Arroyo-Estrada JF, Cruz-Ortega R (2002) The role of caffeine in the production decline due to auto toxicity in coffee and tea production. In: Reigosa MJ, Pedrol N (eds) Allelopathy from molecules to ecosystems. Science Publishers Inc., Enfiled, pp 71–92
Andrzej B, Hayat S (2009) Effect of brassinosteroids on the plant responses to the environmental stresses. Plant Physiol Biochem 47:1–8
Anjum T, Bajwa R (2005) A bioactive annuionone from sunflower leaves. Phytochemistry 66:1919–1921
Asao T, Kitawaza H, Tomita K, Suyama K, Yamamoto K, Hosoki T, Pramanik MHR (2004) Mitigation of cucumber autotoxicity in hydroponic culture using microbial strain. Sci Hort 99:207–214
Ashraf M, Athar HR, Harris PJC, Kwon TR (2008) Some prospective strategies for improving crop salt tolerance. Adv Agron 97:45–110
Aslam F, Khaliq A, Tanveer A, Zahir ZA, Matloob A (2016) Wheat residue incorporation modulate emergence and seedling growth of canary grass by affecting biochemical attributes and soil properties. Int J Agric Biol 18:1033–1042
Bacilio-Jimenez M, Aguilar-Flores S, Ventura-Zapata E, Perez-Campos E, Bouquelet S, Zenteno E (2003) Chemical characterization of root exudates from rice (Oryza sativa) and their effects on the chemotactic response of endophytic bacteria. Plant Soil 249:271–277
Baerson SR, Sanchez-Moreiras AM, Pedrol-Bonjoch N, Schulz M, Kagan IA, Agarwal AK, Reigosa MJ, Duke SO (2005) Detoxification and transcriptome response in Arabidopsis seedlings exposed to the allelochemical benzoxazolin-2(3H)-one. J Biol Chem 280:21867–21881
Bais HP, Weir TL, Perry LG, Gillroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and microorganisms. Annu Rev Plant Biol 57:233–266
Barkosky RR, Einhellig FA (1993) Effects of salicylic acid on plant water relationships. J Chem Ecol 19:237–247
Barkosky RR, Einhellig FA (2003) Allelopathic interference of plant–water relationships by para-hydroxybenzoic acid. Bot Bull Acad Sinica 44:53–58
Barkosky RR, Butler JL, Einhellig FA (2000) Caffeic acid-induced changes in plant water relationships and photosynthesis in leafy spurge. J Chem Ecol 26:2095–2109
Barnes JP, Putnam RA (1983) Rye residues contribute weed suppression in no-tillage cropping systems. J Chem Ecol 9:1045–1057
Barnes JP, Putnam AR, Burke BA, Aasen AJ (1987) Isolation and characterization of allelochemicals in rye herbage. Phytochemistry 26:1385–1390
Basra SMA, Ali Z, Munir H, Mahmood A, Yousaf S (2011) Mitigation of drought stress in maize by natural and synthetic growth promoters. J Agric Soc Sci 7:56–62
Batish DR, Singh HP, Kaur S (2001) Crop allelopathy and its role in ecological agriculture. J Crop Prod 4:121–161
Batish DR, Singh HP, Setia N, Kaur S, Kohli RK (2006) 2-Benzoxazolinone (BOA) induced oxidative stress, lipid peroxidation and changes in some antioxidant enzyme activities in mung bean (Phaseolus aureus). Plant Physiol Biochem 44:819–827
Baziramakenga R, Simard RR, Leroux GD (1994) Effects of benzoic and cynamic acids on growth, mineral composition and chlorophyll contents of soybean. J Chem Ecol 20:2821–2833
Baziramakenga R, Leroux GD, Simard RR (1995) Effects of benzoic and cynamic acids on membrane permeability of soybean root. J Chem Ecol 21:1271–1285
Baziramakenga R, Leroux GD, Simard RR, Nadeau P (1997) Allelopathic effects of phenolic acids on nucleic acid and protein levels in soybean seedlings. Can J Bot 75:445–450
Belz RG (2014) Is hormesis an underestimated factor in the development of herbicide resistance? 26th German Conference on weed Biology an Weed Control, March 11–13, 2014, Braunschweig, Germany. pp: 81–91. doi:10.5073/jka.2014.443.009)
Belz R, Hurle K (2004) Is there a benzoxazinone-mediated potential for weed-suppression in Triticum astivum L. spp. and Secale cereale L. In: Proceedings of Second European Allelopathy Symposium-Allelopathy: From Understanding to Application. Pulawy, Poland, 4 June 2004. pp 98
Belz RG, Hurle K (2005) Differential exudation of two benzoxazinoids-one of the determining factors for seedling allelopathy of Triticeae species. J Agric Food Chem 53:250–261
Belz RG, Cedergreen N, Duke SO (2011) Herbicide hormesis–can it be useful in crop production? Weed Res 51:321–332
Bergsma-Vlami M, Prins ME, Raaijmakers JM (2005) Influence of plant species on population dynamics, genotypic diversity and antibiotic production in the rhizosphere by indigenous Pseudomonas spp. FEMS Microbiol Ecol 52:59–69
Bertholdsson NO (2004) Variation in allelopathic activity over 100 years of barley selection and breeding. Weed Res 44:78–86
Bertholdsson NO (2005) Early vigour and allelopathy-Two useful traits for enhanced barley and wheat competitiveness with weeds. Weed Res 45:94–102
Bertholdsson NO (2010) Breeding spring wheat for improved allelopathic potential. Weed Res 50:49–57
Bertholdsson N-O (2011) Use of multivariate statistics to separate allelopathic and competitive factors influencing weed suppression ability in winter wheat. Weed Res 51:273–283
Bertholdsson NO (2012) Allelopathy-A tool to improve the weed competitive ability of wheat with herbicide-resistant black grass (Alopecurus myosuroides Huds.). Agron J 2:284–294
Bertholdsson N-O, Andersson SC, Merker A (2012) Allelopathic potentials of Triticum spp., Secale spp., Triticosecale spp. and use of chromosome substitutions and translocations to improve weed suppression ability in winter wheat. Plant Breeding 131:75–80
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256:67–83
Bertin C, Weston LA, Tengfang W, Georg HJ, Thomas O (2007) Grass roots chemistry: meta-tyrosine, an herbicidal nonprotein amino acid. PNAS 104:16964–16969
Bertin C, Weston LA, Kaur H (2008) Allelopathic crop development: molecular and traditional plant breeding approaches. Plant Breed Rev 30:231–258
Bhowmik PC, Inderjit (2003) Challenges and opportunities in implementing allelopathy for natural weed management. Crop Prot 22:661–667
Biprodukt (1984) Agrostemin-gift of nature. Novi Dani, Beograd
Birkett MA, Chamberlain K, Hooper AM, Pickett JA (2001) Does allelopathy offer real promise for practical weed management and for explaining rhizosphere interactions involving higher plants? Plant Soil 232:31–39
Blum U (1998) Effects of microbial utilization of phenolic acids and their phenolic acid breakdown products on allelopathic interactions. J Chem Ecol 24:685–708
Blum U, Shafer SR (1988) Microbial population and phenolic acids in soil. Soil Biol Biochem 20:793–800
Blum U, Wentworth TR, Klein K, Worshman AD, King LD, Gerig TM, Lyu SW (1991) Phenolic acid contents of soil from wheat no-till soil, wheat-conventional till, and fallow-conventional till soybean cropping system. J Chem Ecol 17:1045–1068
Blum U, Wentworth TR, Klein K, Worshman AD, Holappa LD, King LD (1992) Allelopathy in wheat-conventional and wheat no-till soils, developments of soil extract bioassays. J Chem Ecol 18:2191–2221
Blum U, Shafer SR, Lehman ME (1999) Evidence for inhibitory allelopathic interactions involving phenolic acids in field soils: concepts vs. an experimental model. Crit Rev Plant Sci 18:673–693
Blum U, King LD, Brownie C (2002) Effects of wheat residues on dicotyledonous weed emergence in a simulated no-till system. Allelopathy J 9:159–176
Bredenberg JB, Honkanen E, Virtanen AI (1962) The kinetics and mechanism of the decomposition of 2,4-dihydroxy-1,4-benzoxazin-3-one. Acta Chem Scand 16:135–141
Brimecombe MJ, Lelj FAD, Lynch JM (2001) The Rhizosphere. The Effect of Root Exudates on Rhizosphere Microbial Populations. In: Pinton R, Varanini Z, Nannipieri P (ed) The Rhizosphere. Biochemistry and Organic Substances at the Soil-Plant Interface. Marcel Dekker, New York, pp 95–140
Brooks AM (2008) Allelopathy in rye (Secale cereal). Master’s Thesis, Dept Crop Sci, North Carolina State University
Burgos NR, Talbert RE, Mattice JD (1999) Cultivar and age differences in the production of allelochemicals by Secale cereale. Weed Sci 47:481–485
Calabrese EJ (2005) Paradigm lost, paradigm found: the re-emergence of hormesis as a fundamental dose response model in the toxicological sciences. Environ Pollut 138:378–411
Calabrese EJ, Blain R (2005) The occurrence of hormetic dose-responses in the toxicological literature, the hormesis database: an overview. Toxicol Appl Pharmacol 202:289–301
Cedergreen N, Ritz C, Streibig JC (2005) Improved empirical models describing hormesis. Environ Toxicol Chem 24:3166–3172
Chase WR, Nair MG, Putnam AR, Mishra SK (1991) 2, 2¢- oxo-1, 1¢-azobenzene: microbial transformation of rye (Secale cereale L.) allelochemicals in field soils by Acinetobacter calcoaceticus: III. J Chem Ecol 17:1575–1584
Cheema ZA, Khaliq A, Hussain R (2003) Reducing herbicide rate in combination with allelopathic sorgaab for weed control in cotton. Int J Agric Biol 5:4–6
Cheng F, Cheng Z (2015) Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 6:1020. doi:10.3389/fpls.2015.01020
Chiapusio G, Pellissier F, Gallet C (2004) Uptake and translocation of phytochemical 2-benzoxazolinone (BOA) in radish seeds and seedlings. J Exp Bot 55:1587–1592
Chobot V, Hadacek F (2009) Milieu-dependent pro- and antioxidant activity of juglone may explain linear and nonlinear effects on seedling development. J Chem Ecol 35:383–390
Chon SU, Kim YM (2004) Herbicidal potential and quantification of suspected allelochemicals from four grass crop extracts. J Agron Crop Sci 190:145–150
Chon SU, Kim YM, Lee JC (2003a) Herbicidal potential and quantification of causative allelochemicals from several compositae weeds. Weed Res 43:444–450
Chon SU, Nelson CJ, Coutts JH (2003b) Physiological assessment and path coefficient analysis to improve evaluation of alfalfa autotoxicity. J Chem Ecol 29:2395–2406
Chou CH (1992) Allelopathy in relation to agricultural productivity in Taiwan: problems and prospects. In: Rizvi SJH, Rizvi V (eds) Allelopathy: Basic and Applied Aspects. Chapman and Hall Press, London, pp 179–200
Chou CH (1999) Roles of allelopathy in plant biodiversity and sustainable agriculture. Crit Rev Plant Sci 18:609–636
Chou CH, Chiou SJ (1979) Auto intoxication mechanism of Oryza sativa II. Effects of culture treatments on the chemical nature of paddy soil on rice productivity. J Appl Ecol 5:839–859
Chou CH, Patrick ZA (1976) Identification and phytotoxic activity of compounds produced during decomposition of corn and rye residues in soil. J Chem Ecol 2:369–387
Chum M, Batish DR, Singh HP, Kohli RK (2010) Comparative phytotoxicity of some benzoxazinoides on the early growth of selected weeds. The Bioscan 5:537–540
Copaja SV, Nicol D, Wratten SD (1999) Accumulation of hydroxamic acids during wheat germination. Phytochemistry 50:17–24
Cruz-Ortega R, Anaya AL, Hernandez-Bautista BE (1998) Effects of allelochemical stress produced by sicyosdeppei on seedling root ultrastructure of Phaseolous valgaris and Cucubita ficifolia. J Chem Ecol 24:2039–2057
D’Abrosca B, Scognamiglio M, FiumanoV Esposito A, Choi YH, Verpoorte R et al (2013) Plant bioassay to assess the effects of allelochemicals on the metabolome of the target species Aegilops geniculata by an NMR-based approach. Phytochemistry 93:27–40
Dayan FE, Romagni JG, Duke SO (2000) Investigating the mode of action of natural phytotoxins. J Chem Ecol 26:2079–2094
De Albuquerque MB, Santos RC, Lima LM, Filho PAM, Nogueira RJMC, Amara CAG, Ramos AR (2011) Allelopathy, an alternative tool to improve cropping systems, A review. Agron Sustain Dev 31:379–395
Delhaize E, Ryan PR, Randall PJ (1993) Aluminium tolerance in wheat (Triticum aestivum L.) II. Aluminium stimulated excretion of malic acid from root apices. Plant Physiol 103:695–702
Devakumar C, Parmar BS (1993) Pesticides of higher plant and microbialorigin. In: Parmar BS, Devakumar C (eds) Botanical and Pesticides. SPS Publication No. 4, Society of Pesticide Science, India and Westvill Publishing House, New Delhi. pp 1–73
Devi SR, Prasad MNV (1992) Effect of ferulic acid on growth and hydrolytic enzyme activities of germinating maize seeds. J Chem Ecol 18:1981–1990
Ding H, Lamb RJ, Ames N (2000) Inducible production of phenolic acids in wheat and antibiotic resistance to Sitodiplosis mosellana. J Chem Ecol 26:969–985
Dong LY, Wang MH, Wu SW, Shen JL (2005) Isolation and identification of allelochemicals from wheat and allelopathy on Leptochloa chinensis in direct-seeding rice field. Chin J Rice Sci 19:551–555
Duke SO (2015) Proving Allelopathy in Crop-Weed Interactions. Weed Sci Special issue: 121–132
Duke SO, Scheffler BE, Dayan FE, Weston LA, Ota E (2001) Strategies for using transgenes to produce allelopathic crops. Weed Technol 15:826–834
Duke SO, Dayan FE, Rimando AM, Schrader KK, Aliotta G, Oliva A, Romagni JG (2002) Chemicals from nature for weed management. Weed Sci 50:138–151
Duke SO, Cedergreen N, Velini ED, Belz RG (2006) Hormesis: is it an important factor in herbicide use and allelopathy? Outlooks Pest Manag 17:29–33
Einhellig FA (1986) Mechanisms and mode of action of allelochemicals. In: Putnum AR, Tang CS (eds) The sciences of allelopathy. Wiley, New York, pp 171–188
Einhellig FA (1995) Mechanism of action of allelochemicals in allelopathy. In: Inderjit, Dakshini MM, Einhellig FA (ed) Allelopathy: organisms, processes and applications. ACS, Washington D C, pp 96-116
Einhellig FA (1996) Interactions involving allelopathy in cropping systems: allelopathy in cropping systems. Agronomy J. 88:883–893
Einhellig FA (2004) Mode of allelochemical action of phenolic compounds. In: Macias FA, Galindo JCG, Molinilo JMG, Cutler HG (eds) Allelopathy: chemistry and mode of action of allelochemicals. CRC Press, Boca Raton, pp 217–238
Einhellig FA, Muth MS, Schon MK (1985) Effects of allelochemicals on plant-water relationship. In: Thompson AC (ed) The chemistry of allelopathy. American Chemical Society, Washington, D.C., pp 170–195
Farooq M, Jabran K, Cheema ZA, Wahid A, Siddique KHM (2011) The role of allelopathy in agricultural pest management. Pest Manage Sci 67:493–506
Ferguson JJ, Rathinasabapathi B, Reichenbach R (2003) Evaluation of allelopathic potential of wood chips used for weed suppression in sustainable fruit crops production systems. Annual meeting of the American Society of Horticultural Science, Providence, RI, Oct 3–6, 2003
Fomsgaard IS, Mortensen AG, Carlsen SCK (2004) Microbial transformation products of benzoxazolinone and benzoxazinone allelochemicals-a review. Chemosphere 54:1025–1038
Fragasso M, Iannucci A, Papa R (2013) Durum wheat and allelopathy: toward wheat breeding for natural weed management. Front Plant Sci 4:375. doi:10.3389/fpls.2013.00375
Franche C, Lindström K, Elmerich C (2009) Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 321:35–59
Frey M, Huber K, Park WJ, Sicker D, Lindberg P, Meeley RB, Simmons CR, Yalpani N, Gierl A (2003) A 2-oxoglutarate-dependent dioxygenase is integrated in DIMBOA-biosynthesis. Phytochemistry 62:371–376
Frey M, Schullehner K, Dick R, Fiesselmann A, Gierl A (2009) Benzoxazinoid biosynthesis, a model for evolution of secondary metabolic pathways in plants. Phytochemistry 70:1645–1651
Friebe A, Roth U, Kuck P, Schnabl H, Schulz M (1997) Effects of 2,4-dihydroxy-1, 4-benzoxazin-3-ones on the activity of plasma membrane H+-ATPase. Phytochemistry 44:979–983
Friebe A, Vilich V, Hennig L, Kluge M, Sicker D (1998) Detoxification of benzoxazolinone allelochemicals from wheat by Gaeumannomyces graminis var tritici, G. graminis var. graminis, G. graminis var. avena and Fusarium culmorum. Appl Environ Microbiol 64:2386–2391
Friedman J, Waller GR (1983) Caffeine hazards and their prevention in germinating seeds of coffee (Coffea arabica L.). J Chem Ecol 9:1099–1106
Gagliardo RW, Chilton WS (1992) Soil transformation of 2(3H)-benzoxazolone of rye into phytotoxic 2-amino-3Hphenoxazin- 3-one. J Chem Ecol 18:1683–1691
Gaspar EMM, Pereira M, Chaves das Neves HJ (1999a) Potential allelopathic sterols and keto-steroids from wheat straw (Triticum aestivum). In: Macias FA, Galindo JCG, Molinillo JMG, Cutler HG (eds) Recent Advances in Allelopathy. Servicio De Publicaciones -Universidad de Cadiz, Cadiz, Spain, pp 69–80
Gaspar EMM, Pereira M, Chaves das Neves HJ (1999) Potential allelopathic sterols and ketosteroids from wheat straw (Triticum aestivum). In: Macias FA, Galindo JCG, Molinillo JMG, Cutler HG (eds.) Recent Advances in Allelopathy, Vol. 1, Servicio De Publicaciones-Universidad de Cadiz, Cadiz, Spain. pp 69–80
Gealy DR, Yan W (2012) Weed suppression potential of‘Rondo’ and other Indica rice germplasm lines. Weed Technol 26:517–524
Gent MPN, Parrish ZD, White JC (2005) Nutrient uptake amongst the sub species of Cucurbita pepo L. is related to exudation of citric acid. J Amer Soc Hort Sci 130:782–788
Gerald FL, Blum UB, Fiscus EL (1992) Short-term effects of ferulic acid anion uptake and water relations in cucumber seedlings. J Exp Bot 43:649–655
Gilbert GA, Knight JD, Allan DL, Vance CP (1999) Acid phosphatase activity in phosphorus deficient white lupin roots. Plant Cell Environ 22:801–810
Givovich A, Niemeyer HM (1995) Comparison of the effect of hydroxamic acids from wheat on five species of cereal aphids. Entomol Exp Appl 74:115–119
Gniazdowska A, Bogatek R (2005) Allelopathic interactions between plants multi-site action of allelochemicals. Acta Physiol Plantarum 27:395–407
Gniazdowska A, Dobrzynska U, Babanczyk T, Bogatek R (2007) Breaking the apple embryo dormancy by nitric oxide involves the stimulation of ethylene production. Planta 225:1051–1057
Gordon-Weeks R, Pickett JA (2009) Role of natural products in nature: plant–insect interactions. In: Osborn AE, Lanzotti V (eds) Plant-derived natural products. Springer, Berlin, pp 321–347
Grayston SJ, Wang S, Cambell CD, Edwards AC (1998) Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol Biochem 30:369–378
Guenzi WD, McCalla TM (1966) Phenolic acids in oat, wheat, sorghum and corn residues and their phytotoxicity. Agron J 58:303–304
Guenzi WD, McCalla TM, Norstadt FA (1967) Presence and persistence of phytotoxic substances in wheat, oat, corn and sorghum residues. Agron J 59:163–165
Gulzar A, Siddiqui MB, Shazia BI (2014) Assessment of allelopathic potential of Cassia sophera L. on seedling growth and physiological basis of weed plants. Afr J Biotech 13:1037–1046
Gurusiddaiah S, Weller DM, Sarkar A, Cook RJ (1986) Characterization of antibiotic produced by a strain of Pseudomonas fluorescens inhibitory to Gaeumannomyces graminisvar tritici and Pythium spp. Antimicrob Agents Chemother 29:488–495
Haider K, Martin JP, Rietz E (1977) Decomposition in soil of14C-labeled coumaryl alcohols; free and linked into dehydropolymer and plant lignins and model humic acids. Soil Sci Soc Am J 41:556–562
Haig T, Pratley J, An M, Haig T, Hildebrand S (2005) Using allelopathy to search for new natural herbicides from plants. In: Harper JDI, An M, Wu H, Kent JH (eds) Proceedings of the 4th World Congress on Allelopathy. Charles Sturt University, Wagga, pp 565–568
Hairston JE, Sanford JO, Pope DF, Horneck DA (1987) Soybean-wheat double cropping, implications from straw management and supplemental nitrogen. Agron J 79:281–286
Harpers HT, Lynch JM (1981a) The kinetics of straw decomposition in relation to its potential to produce the phytotoxin acetic acid. J Soil Sci 32:627–637
Harpers HT, Lynch JM (1981b) The chemical components and decomposition of wheat straw leaves, internodes and nodes. J Sci Food Agric 32:1057–1062
Hatcher PE, Melander B (2003) Combining physical, cultural and biological methods prospects for integrated non-chemical weed management strategies. Weed Res 43:303–322
Hayashi H, Czaja I, Lubenow H, Schell J, Walden R (1992) Activation of a plant gene by T-DNA tagging: auxin-independent growth in vitro. Science 258:1350–1353
Holappa LD, Blum U (1991) Effects of exogenously applied ferulic acid, a potential allelo-pathic compound, on leaf growth, water utilization and endogenous abscisic acid levels of tomato, cucumber and bean. J Chem Ecol 17:865–886
Huang Z, Haig T, Wu H, An M, Pratley J (2003) Correlation between phytotoxicity on annual ryegrass (Lolium rigidum) and production dynamics of allelochemicals within root exudates of an allelopathic wheat. J Chem Ecol 29:2263–2279
Hussain MI, González L, Reigosa MJ (2010) Phytotoxic effect of allelochemicals and herbicides on photosynthesis, growth and carbon isotope discrimination in Lactuca sativa. Allelopathy J 26:157–174
Inderjit (2001) Soil environment effects on allelochemical activity. Agron J 93:79–84
Inderjit (2005) Soil microorganisms: an important determinant of allelopathic activity. Plant Soil 274:227–236
Inderjit, Keating KI (1999) Allelopathy: principles, procedures, processes, and promises for biological control. Adv Agron 67:141–231
Inderjit Streibig JC, Olofsdotter M (2002) Joint action of phenolic acids mixtures and its significance in allelopathy research. Physiol Plant 114:422–428
Iqbal J, Cheema ZA (2007) Intercropping of field crops in cotton for management of purple nutsedge (Cyperus rotundus L.). Plant Soil 300:163–171
Jabran K, Farooq M, Aziz T, Siddique KHM (2012) Allelopathy and crop nutrition. In: Cheema, ZA, Farooq M, Wahid A (eds) Allelopathy: current trends and future applications. Springer: Verlag Berlin, pp 113–143
Jabran K, Mahajan G, Sardana V, Chauhan BS (2015) Allelopathy for weed control in agricultural systems. Crop Prot 72:57–65
Javed K (2011) Impact of allelopathy of sunflower (Helianthus annus L.) roots extract on physiology of wheat (Triticum aestivum L.). AJB 10:14465–14477
Javed N, Gowen SR, Inam-ul-Haq M, Abdullah K, Shahina F (2007) Systemic and persistent effect of neem (Azadirachta indica) formulations against root-knot nematodes, Meloidogyne javanica and their storage life. Crop Prot 26:911–916
Jia C, Kudsk P, Mathiassen SK (2006) Joint action of benzoxazinone derivatives and phenolic acids. J Agric Food Chem 54:1049–1057
Jimanez MB, Flores SA, Zapata EV, Campos EP, Bouquelet S, Zenteno E (2003) Chemical characterization of root exudates from rice (Oryza sativa) and their effect on the chemotactic response of endophytic bacteria. Plant Soil 249:271–277
Karmarkar SV, Tabatabai MA (1991) Effects of biotechnology byproducts and organic acids on nitrification in soils. Biol Fertil Soils 12:165–169
Kato-Noguchi H, Ino T (2005) Possible involvement of momilactone B in rice allelopathy. J Plant Physiol 162:718–721
Kennedy AC (1999) Soil microorganisms for weed management. J Crop Prod 2:123–138
Khaliq A, Matloob A, Aslam F, Khan MB (2011) Influence of wheat straw and rhizosphere on seed germination, early seedling growth and biochemical attributes of Trianthema portulacastrum. Planta Daninha 29:523–533
Khaliq A, Matloob A, Aslam F, Mushtaq MN, Khan MB (2012a) Toxic action of aqueous wheat straw extract on horse purslane. Planta Daninha 30:269–278
Khaliq A, Matloob A, Riaz Y (2012b) Bio-economic and qualitative impact of reduced herbicide use in direct seeded fine rice through multipurpose tree water extracts. Chilean J Agric Res 72:350–357
Khaliq A, Matloob A, Saqib M, Wahid A (2013) Seed pre-treatments help improve maize performance under sorghum allelopathic stress. J Crop Improv 27:586–605
Khaliq A, Matloob M, Hussain A, Hussain S, Aslam F, Zamir SI, Chattha MU (2015) Wheat residue management options affect productivity, weed growth and soil properties in direct-seeded fine aromatic rice. Clean Air Water Environ (In press)
Khanh TD, Chung MI, Xuan TD, Tawata S (2005) The exploitation of crop allelopathy in sustainable agricultural production. J Agron Crop Sci 191:172–184
Kim HK, Choi YH, Verpoorte R (2011) NMR-based metabolomics:where do we stand, where do we go? Trends Biotechnol 29:267–275
Kimber RWL (1967) Phytotoxicity from plant residues. I. The influence of rotted wheat straw on seedling growth. Aust J Agric Res 18:361–374
Koch HJ, Matthiessen A, Baeumer K (1992) On the problem of straw mulch in reduce tillage arable land. Influence of the chopping of wheat straw on the liberation of phytoxins. J Agron Crop Sci 169:298–309
Kohli RK, Singh HP, Batish DR (2001) Allelopathy in Agroecosystems. Food Products Press, New York
Komai K, Sugiwaka Y, Sato S (1981) Plant growth retardant of extracts obtained from water nutgrass (Cyperus serotinus Rottb.). Mem. Fac Agric Kinki Univ 14:57–65 (Chem. Abstr. 45: 16296C)
Kong CH, Li HB, Hu F, Xu XH (2006) Allelochemicals released by rice roots and residues in soil. Plant Soil 288:47–56
Krysan PJ, Young JC, Sussman MR (1999) T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 11:2283–2290
Kumar P, Gagliardo RW, Chilton WS (1993) Soil transformation of wheat and corn metabolites MBOA and DIM2BOA into amino phenoxazinones. J Chem Ecol 19:2453–2461
Labbafi MR, Hejazi A, Meighani F, Khalaj H, Baghestani MA (2008) A Study of the Allelopathic potential of wheat (Triticum aestivum L.) cultivars on the growth of field bindweed (Convolvulus arvensis L.) and Rye (Secale cereale L.). Environ Sci 5:1–10
Labbafi MR, Hejazi A, Maighany F, Khalaj H, Mehrafarin A (2010) Evaluation of allelopathic potential of Iranian wheat (Triticum aestivum L.) cultivars against weeds. Agric Biol J. North America 1:355–361
Labbafy MR, Maighany F, Hejazy A, Khalaj H, Baghestany AM, Allahdady I, Mehrafarin A (2009) Study of allelopathic interaction of wheat (Triticum aestivum L.) and rye (Secale cereal L.) using equal-compartment-agar method. Asian J Agri Sci 1:25–28
Lehman ME, Blum U (1997) Cover crop debris effects on weed emergence as modified by environmental factors. Allelopathy J 4:69–88
Lehman ME, Blum U (1999) Evaluation of ferulic acid uptake as a measurement of allelochemical dose: effective concentration. J Chem Ecol 25:2585–2600
Liebl RA, Worsham AD (1983) Inhibition of morning glory (Ipomoea lacunosa) and certain other weed species by phytotoxic components of wheat (Triticum aestivum L.) straw. J Chem Ecol 9:1027–1044
Lithourgidis AS, Dordas CA, Damalas CA, Vlachostergios DN (2011) Annual intercrop: an alternative pathway for sustainable agriculture. Aust J Crop Sci 5:396–410
Liu Y, Chen X, Duan S, Feng Y, An M (2011) Mathematical modeling of plant allelopathic hormesis based on ecological-limiting-factor models. Dose-Response 9:117–129
Lodhi MAK, Bilal R, Malik KA (1987) Allelopathy in agroecosystems: wheat phytotoxicity and its possible roles in crop rotation. J Chem Ecol 13:1881–1891
Lynch JM (1978) Production and phytotoxicity of acetic acid in anaerobic soils containing plant residues. Soil Bio Biochem 10:131–135
Lynch JM, Gunn KB, Panting LM (1980) On the concentration of acetic acid in straw and soil. Plant Soil 56:93–98
Ma YQ (2005) Allelopathic studies of common wheat (Triticum aestivum L.). Weed Biol Manag 5:93–104
Macias FA, Molinillo JMG, Galindo JCG, Varela RM, Simonet AM, Castellano D (2001) The use of allelopathic studies in the search for natural herbicides. J Crop Prod 4:237–255
Macias FA, Ascension T, Galindo JLG, Rosa M, Varela AJ, Molinillo JMG (2002) Bioactive terpenoids from sunflower leaves cv. Peredovick. Phytochemistry 61:687–692
Macias FA, Marin D, Oliveros-Bastidas A, Castellano D, Simonet AM, Molinillo JMG (2006) Structure-activity relationship (SAR) studies of benzoxazinones, their degradation products and analogues phytotoxicity on problematic weeds Avena fatua L. and Lolium rigidum Gaud. J Agric Food Chem 54:1040–1048
Mahmood K (2013) Evaluation of allelopathic potential of some indigenous wheat (Triticum aestivum L.) genotypes. Ph.D. Thesis, Dept Agron, University of Agriculture, Faisalabad, Pakistan
Mahmood K, Khaliq A, Cheema ZA, Arshad M (2013) Allelopathic activity of Pakistani wheat genotypes against wild oat (Avena fatua). Pak J Agr Sci 50:169–176
Makkar HPS, Becker K (1996) Nutritional value and antinutritional components of whole and ethanol extracted Moringa olifera leaves. Anim Feed Sci Tech 63:211–228
Maqbool N (2010) Exploring the role of sorgab in improving water stress tolerance in maize at germination and vegetative growth stages. M. Phil Thesis, Department of Botany, University of Agriculture, Faisalabad, Pakistan
Maqbool N, Wahid A, Farooq M, Cheema ZA, Siddique KHM (2012) Allelopathy and abiotic stress interaction in crop plants. In: Cheema ZA, Farooq M, Wahid A (eds) Allelopathy: current trends and future applications. Springer, Germany, pp 113–143
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
Martin JP, Haider K (1976) Decomposition of specifically carbon-14-labelled ferulic acid free and linked into model humic acid-type polymer. Soil Science Society of America, Proceedings 36:311–315
Mathiassen SK, Kudsk P, Mogensen BB (2006) Herbicidal effects of soil-incorporated wheat. J Agric Food Chem 54:1058–1063
Matloob A, Khaliq A, Farooq M, Cheema ZA (2010) Quantification of allelopathic potential of different crop residues for the purple nutsedge suppression. Pak J Weed Sci Res 16:1–10
Maugh TH (1981) New chemicals promise larger crops Sci 212:33–34
Mayton HS, Olivier C, Vaughn SF, Loria R (1996) Correlation of fungicidal activity of Brassica species with allyl isothiocyanate production in macerated leaf tissue. J Phytopathol 86:267–271
Mersie W, Singh M (1993) Phenolic acids affect photosynthesis and protein synthesis by isolated leaf cells of velvet-leaf. J Chem Ecol 19:1293–1301
Miri HR (2011) Allelopathy of 68 Iranian wheat genotypes released between 1939 and 2009. Asian J Agric Sci 3:462–468
Mogensen BB, Krongaard T, Mathiassen SK, Kudsk P (2006) Quantification of benzoxazinones in wheat (Triticum aestivum) varieties grown under contrasting conditions in Denmark. J Agric Food Chem 54:1023–1030
Mohamadi N, Rajaie P (2009) Effects of aqueous eucalyptus (E. camadulensis Labill) extracts on Seed germination, seedling growth and physiological responses of Phaseolus vulgaris and Sorghum bicolor. Asian J Plant Sci 4:1292–1296
Molisch H (1937) Der Einfluss einer Pflanze auf die andere—Allelpathie. Fisher, Jena
Morant AV, Jørgensen K, Jørgensen C, Paquette SM, Sánchez-Pérez R, Møller BL, Bak S (2008) & #x03B2;-Glucosidases as detonators of plant chemical defense. Phytochemistry 69:1795–1813
Muminovic S (1991) Allelopathic effect of straw of crops on growth of weeds. Savremena-Poljoprivreda 39:27–30
Munir R (2011) Evaluating the role of allelopathy in improving the resistance against heat and drought stresses in wheat. MSc Thesis, Department of Agronomy, University of Agriculture, Faisalabad, Pakistan
Muscolo A, Sidari M, Santonoceto C, Santis C (2004) Kikuyu grass: effects of salinity and acidity on growth, biochemistry and root morphology. Recent Res Develop Agron Hort 1:89–101
Nair MG, Whiteneck CJ, Putnam AR (1990) 2, 2¢-oxo-l, 1¢-azobenzene, a microbially transformed allelochemical from 2, 3-benzoxazorinone I. J Chem Ecol 16:353–364
Nardi S, Concheri G, Pizzeghello D, Sturaro A, Rella R, Parvoli G (2000) Soil organic matter mobilization by root exudates. Chemosphere 5:653–658
Narwal SS (1994) Allelopathy in crop production. Scientific publishers, Jodhpur, p 288
Narwal SS (1998) Allelopathic strategies for weed management in rice-wheat rotation in north-western India. In: Olofsdotter M (ed) Workshop on allelopathy in rice (Manila, Philippines, 25-27 November 1996. IRRI, Los Banos, Philippines, pp 117–131
Narwal SS (1999) Allelopathy in weed management’. In: Narwal SS (ed) Allelopathy update, basic and applied aspects. Science Publishers Inc, Enfield, pp 203–254
Narwal SS (2000) Weed management in rice: wheat rotation by allelopathy Critic Rev. Plant Sci 19:249–266
Narwal SS (2006) Allelopathy in ecological sustainable agriculture. In: Reigosa MJ, Pedrol N, González L (eds) Allelopathy: a physiological process with ecological implications. Springer, Dordrecht, Netherlands, pp 537–564
Narwal SS, Sarmah MK (1996) Effect of wheat residues and forage crops on the germination and growth of weeds. Allelopathy J 3:229–240
Niemeyer HM (1988) Hydroxamic acid content of triticum species. Euphytica 37:289–293
Niemeyer HM, Jerez JM (1997) Chromosomal location of genes for hydroxamic acid accumulation in Triticum aestivum L. (wheat) using wheat aneuploids and wheat substitution lines. Heredity 79:10–14
Niemeyer HM, Perez FJ (1995) Potential of hydroxamic acids in the control of cereal pests, disease, and weeds. In: Inderjit, Dakshini KMM, Einhellig FA (ed) Allelopathy: organisms, processes, and applications, ACS Symposium Series No. 582, American Chemical Society, Washington, D.C, pp 261–270
Niemeyer HM, Pesel E, Copaja SV, Bravo HR, Franke S, Francke W (1989) Change in hydroxyamic acid levels of wheat plants induced by aphid feeding. Phytochemistry 28:447–449
Nikus J, Jonsson LMV (1999) Tissue localization of β-glucosidase in rye, maize and wheat seedlings. Physiol Plantarum 107:373–378
Nomura T, Ishihara A, Imaishi H, Endo TR, Ohkawa H, Iwamura H (2002) Molecular characterization and chromosomal localization of cytochrome P450 genes involved in the biosynthesis of cyclic hydroxamic acids in hexaploid wheat. Mol Genet Genom 267:210–217
Norstadt FA, McCalla TM (1963) Phytotoxic substances from a species of Penicillium. Science 140:410–411
Oerke EC, Dehne HW (2004) Safeguarding production-losses in major crops and the role of crop protection. Crop Prot 23:275–285
Olofsdotter M (1998) Allelopathy for weed control in organic farming. In: Sustainable agriculture for food, energy and industry, pp 453–457
Olofsdotter M, Navarez D, Rebulanan M, Streibig JC (1999) Weed suppressing rice cultivars–does allelopathy play a role? Weed Res 39:441–454
Olofsdotter M, Jensen LB, Courtois B (2002) Improving crop competitive ability using allelopathy-an example from rice. Plant Breed 121:1–9
Penuelas J, Ribas-Carbo M, Giles L (1996) Effect of allelochemicals on plant respiration and oxygen isotopes fractionation by alternative oxidase. J Chem Ecol 22:801–805
Perez FJ (1990) Allelopathic effect of hydroxamic acid from cereal on Avena sativa and A. fatua. Phytochemistry 29:773–776
Peters NK, Frost JW, Long SR (1986) A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 233:977–980
Pinton R, Varanini Z, Nannipieri P (2001) The rhizosphere, biochemistry and organic substances at the soil-plant interface. Marcel Dekker, New York
Prasanta C, Bhownik C, Inderjit (2003) Challenges and opportunities in implementing allelopathy for natural weed management. Crop Prot 22:661–671
Rad U, Hüttl R, Lottspeich F, Gierl A, Frey M (2001) Two glucosyltransferases are involved in detoxification of benzoxazinoids in maize. Plant J 28:633–642
Rajput P, Rao PB (2013) Effect of different wheat straw extracts on germination and growth of three dominant weed species. Int J Bot 3:71–78
Rambakudzibga AM (1991) Allelopathic effect of aqueous wheat (Triticum aestivum L.) straw extract on the germination of eight arable weeds commonly found in Zimbabwe, Zimbawe. J Agri Res 29:77–79
Reese JC (1979) Interaction of allelochemicals with nutrients in herbivore food. In: Rosenthal GP, Janzen DH (eds) Herbivores: their interaction with secondary plant metabolites. Academic Press, New York, pp 309–330
Reigosa MJ, Sánchez-Moreiras A, González L (1999) Ecophysiological approach in allelopathy. Crit Rev Plant Sci 18:577–608
Reigosa M, Gomes AS, Ferreira AG, Borghetti F (2013) Allelopathic Research in Brazil. Acta Botanica Brasilica 27:629–646
Ren H, Song T, Wu TQ, Sun L, Liu YX, Yang F, Chen ZY, Dong H (2006) Effects of a biocontrol bacterium on growth and defence of transgenic rice plants expressing a bacterial type- III effector. Ann Microbiol 56:281–287
Rice FEL (1984) Allelopathy, 2nd edn. Academic Press, London, pp 309–316
Rizvi SJH, Rizvi V (1992) Exploitation of allelochemicals in improving crop productivity. In: Rizvi SJH, Rizvi V (eds) Allelopathy: basic and applied aspects. Champan and Hall, London, pp 443–472
Rizvi SJH, Ketata H, Bazazi D, Roostaii M, Pala M (2004) Weed suppressing ability of bread wheat genotypes under greenhouse and field conditions. In: Proceedings of Second European Allelopathy Symposium-Allelopathy: From Understanding to Application. Pulawy, Poland, June 4, 2004. pp 25
Sànchez-Moreiras AM, Reigosa MJ (2005) Whole plant response of lettuce after root exposure to BOA (2(3 H)-benzoxazolinones). J Chem Ecol 31:2689–2703
Sànchez-Moreiras AM, Weiss O, Reigosa MJ (2004) Allelopathic evidence in Poaceae. Bot Rev 69:300–319
Sangeetha C, Baskar P (2015) Allelopathy in weed management: a critical review. Afr J Agric Res 10:1004–1015
Sarwar M, Kremer RJ (1995) Determination of bacterially derived auxin using a microplate method. Lett Appl Microbiol 20:282–285
Schmaidt SK, Ley RE (1999) Microbial competition and low bioavailability limit the expression of allelochemicals in natural soils. In: Dakshini KMM, Foy CL (eds) Inderjit. Allelochemical Interactions. CRC Press Boca Raton Florida, Principles and Practices in Plant Ecology, pp 339–351
Schoonhoven LM, Van Loon JJA, Dicke M (2005) Insect–plant biology. From physiology to evolution. London (UK), Chapman & Hall
Sheteawi SA, Tawfik KM (2007) Interaction. Effect of some biofertilizers and irrigation water regime on mung bean (Vigna radiata) growth and yield. J. Applied Sci. Res. 3:251–262
Shilling DG, Liebl RA, Worsham AD (1985) Rye (Secale cereale) and wheat (Triticum aestivum) mulch: The suppression of certain broad leaf weeds and the isolation and identification of phytotoxins. In: Thompson AC (ed) The Chemistry of Allelopathy: Biochemical Interaction Among Plants. ACS Symposium Series 268. Washington, DC: American Chemical Society, pp 243–271
Singh S, Kapoor KK (1999) Inoculation with phosphate-solublizing microorganisms and a vesicular-arbuscular mycorrhizal fungus improves dry matter yield and nutrient uptake by wheat grown in sandy soil. Biol Fertil Soils 28:139–144
Singh HP, Batish DR, Kohli RK (2001) Allelopathy in agroecosystems: an overview. J Crop Prod 4:1–14
Singh HP, Batish DR, Kohli RK (2003) Allelopathic interactions and allelochemicals: new possibilities for sustainable weed management. Crit Rev Plant Sci 22:239–311
Singh HP, Batish DR, Kaur S, Setia N, Kohli RK (2005) Effects of 2-benzoxazolinone on the germination, early growth and morphogenetic response of mung bean (Paseolus aureus). Annals Appl Biol 147:267–274
Soejima H, Sugiyama T, Ishihara K (1992) Change in cytokinin activities and mass spectrometric analysis of cytokinins in root exudates of rice plant (Oryza sativa L.). Plant Physiol 100:1724–1729
Southam CM, Erlich J (1943) Effects of extract of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathol 33:517–524
Stenlid G (1970) Flavonoids as inhibitors of the formation of adenosine triphosphate in plant mitochondria. Phytochemistry 9:2251–2256
Streibig JC (1980) Models for curve-fitting herbicide dose-response data. Acta Agriculturæ Scandinavia 30:59–64
Streibig JC, Inderjit, Olofsdotter M (2002) Joint action of phenolic acids mixtures and its significance in allelopathy research. Physiol Plant 114:422–428
Subbarao GV, Nakahara K, Ishikawa T, One H, Yoshihashi M, Zhu Y, Zakir HAKM, Deshpande SP, Hash CT, Sahrawwat KL (2013) Biological nitrification inhibition (BNI) activity in sorghum and its characterization. Plant Soil 366:243–259
Swain J (1977) Secondary compounds as protective agents. Ann Rev Plant Physiol 28:79–501
Tang CS, Waiss AC (1978) Short-chain fatty acids as growth inhibitors in decomposing wheat straw. J Chem Ecol 4:225–232
Tang CS, Komai K, Haung RS (1989) In phytochemical ecology: allelochemicals, mycotoxin and insect pheromones and allomones. In: Chou CH, Waller GR (eds) Institute of Academia Sinica Monograph series 9. Taipei, Roc, pp 217–223
Tesfamariam T, Yoshinaga H, Deshpande SP, Rao PS, Sahrawat KL, Ando Y, Nakahara K, Hash CT, Subbarao GV (2014) Biological nitrification inhibition in sorghum: the role of sorgoleone production. Plant Soil 379:325–335
Tesio F, Ferrero A (2011) Allelopathy, a chance for sustainable weed management. Int J Sust Dev World Ecol 17:377–389
Thomashow LS, Weller DM (1988) Role of a phenazine antibiotic from Pseudomonas fluorescensin biological control of Gaeumannomyces graminis var. tritici. J Bacteriol 170:3499–3508
Tranel PJ, Horvath DP (2009) Molecular biology and genomics: new tools for weed science. Bioscience 59:207–215
Tsuzuki E (2001) Application of buckwheat as a weed control. Agric Hortic 76:55–62 (in Japanese)
Tukey HB Jr (1966) Leaching of metabolites from above-ground plant parts and its implications. Bull Torrey Bot Club 93:385–401
Tukey HB (1969) Implication of allelopathy in agriculture plant science. Bot Rev 35:1–16
Understrup AG, Ravnskov S, Hansen HCB, Fomsgaard IS (2005) Biotransformation of 2-benzoxazolinone to 2-amino-phenoxazin-3-one and 2-acetylamino-phenoxazin-3-one in soil. J Chem Ecol 31:1205–1222
Valenzuela H, Smith J (2002) Green manure crops: buck wheat. Sustain. Agric. University of Hawaii at Manoa. http//http://www2.ctahr.hawaii.edu
Villagrasa M, Guillamon M, Labandeira A, Taberner A, Eljarrat E, Barcelo D (2006) Benzoxazinoid allelochemicals in wheat: distribution among foliage, roots, and seeds. J Agric Food Chem 54:1009–1015
Virtanen AI, Hietala PK (1955) 2(3)-Benzoxazplinone, an anti-fusarium factor in rye seedling. Acta Chem Scand 9:1543–1544
Vyvyan JR (2002) Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 58:1631–1636
Wachowska U, Okorski A, Glowacka K (2006) Population structure of microorganisms colonizing the soil environment of winter wheat. Plant soil Environ 52:39–44
Wang F, Wang JG (2005) Study on autotoxic effects of aqueous extracts from eggplant residues. Chin J Econ Agric 13:51–53
Wang G, Yang PR, Zhang H, Ye-Zing L (2004) Effect of returning straw to field on weeds of rice fields and wheat field and the efficiency of chemical weeding. Acta Agri Shanghai 20:87–90
Watanabe K, Ohno N, Yoshioka H, Gershenzon J, Mabry TJ (1982) Sesquiterpene lactones and terpenoids from Helianthus argophyllus. Phytochemistry 21:709–713
Weidenhamer DJ, Romeo JT (2004) Allelochemicals of Polygonella myriophylla: chemistry and soil degradation. J Chem Ecol 30:1061–1078
Weir TL, Park SW, Vivanco JM (2004) Biochemical and physiologi-cal mechanisms mediated by Allelochemicals. Curr Opin Plant Biol 7:472–479
Weston LA (1996) Utilization of allelopathy for weed management in agro-ecosystems. Agron J 88:860–866
Weston LA (2005) History and current trends in the use of allelopathy for weed management. Hort Technol 15:529–534
Weston LA, Duke SO (2003) Weed and crop allelopathy. Crit Rev Plant Sci 22:367–389
Worsham AD (1989) Current and potential techniques using allelopathy as an aid in weed management. In: Chou CH, Waller GR (eds) Phytochemical ecology: allelochemicals, mycotoxins, and insect pheromones and allomones. Institute of Botany, Academia Sinica Monograph Series No. 9, Taipei, Taiwan, pp 275–289
Worthington M, Reberg-Horton C (2013) Breeding cereal crops for enhanced weed suppression: optimizing allelopathy and competitive ability. J Chem Ecol 39:213–231
Wu H, Pratley J, Lemerle D, Haig T, Verbeek B (1998) Differential allelopathic potential among wheat accessions to annual ryegrass. Proceedings of the 9th Australian Agronomy Conference. Wagga Wagga, Australia, pp 567–571
Wu H, Pratley J, Lemerle D, Haig T (1999) Crop cultivars with allelopathic capability. Weed Res 39:171–180
Wu H, Haig T, Pratley J, Lemerle D, An M (2000a) Allelochemicals in wheat (Triticum aestivum L.): variation of phenolic acids in root tissues. J. Agri Food Chem. 48:5321–5325
Wu H, Pratley J, Lemerle D, Haig T (2000b) Evaluation of seedling allelopathy in 453 wheat (Triticum aestivum) accessions against annaul rye grass (Lolium rigidum). Aust J Agric Res 51:937–944
Wu H, Pratley J, Lemerle D, Haig T (2000c) Laboratory screening for allelopathic potential of wheat (Triticum aestivum) accessions against annual ryegrass (Lolium rigidum). Aust J Agric Res 51:259–266
Wu H, Haig T, Pratley J, Lemerle D, An M (2000d) Distribution and exudation of allelochemicals in wheat (Triticum aestivum). J Chem Ecol 26:2141–2154
Wu H, Pratley J, Haig T, Lemerle D, An M (2001a) Allelochemicals in wheat (Triticum aestivum L.): production and exudation of 2, 4-dihydroxy-7-methoxy-1, 4-benzoxazin-3-one. J Chem Ecol 27:1691–1700
Wu H, Pratley J, Lemerle D, Haig T (2001b) Allelopathy in wheat (Triticum aestivum). Ann Appl Biol 139:1–9
Wu H, Pratley J, Lemerle D, Haig T, An M (2001c) Screening methods for the evaluation of crop allelopathic potential. Bot Rev 67:403–415
Wu H, Pratley J, Lemerle D, Haig T (2001c) Wheat allelopathic potential against a herbicide-resistant biotype of annual ryegrass. In: Proceedings of the 10th Australia Agronomy Conference; Australian Society of Agronomy: Hobart, Australia, pp 245–246
Wu H, Pratley J, Haig T (2003a) Phytotoxic effects of wheat extracts on a herbicide-resistant biotype of annual ryegrass (Lolium rigidum). J Agric Food Chem 51:4610–4616
Wu H, Pratley J, Ma W, Haig T (2003b) Quantitative trait loci and molecular markers associated with wheat allelopathy. Theor Appl Genet 107:1477–1481
Wu H, An M, Liu DL, Prately J, Lemerle D (2008) Recent advances in wheat allelopathy. In: Zheng R, Malik MA, Shiming L (eds) Allelopathy in sustainable agriculture and forestry. Springer, New York, pp 235–254
Xuan TD, Tsuzuki E, Tawata S, Khanh TD (2004) Methods to determine allelopathic potential of crop plants for weed control. Allelopathy J 13:149–164
Xuan TD, Tawata S, Khanh TD, Chung IM (2005) Biological control of weeds and plant pathogens in paddy fields by exploiting plant allelopathy: an overview. Crop Prot. 24:197–206
Yaduraj NT, Ahuja KN (1996) Allelopathy. In the illustrated dictionary of weed sciences. In: Yaduraj NT, Ahuja KN (eds) Venus publishing house, 11/298 press colony, Mayapur, New Dehli, India, pp 180
Yang CM, Lee CN, Chou CH (2002) Effects of three allelopathic phenolics on chlorophyll accumulation of rice (Oryza sativa) seedlings: i. Inhibition of supply-orientation. Bot Bull Acad Sinica 43:299–304
Yang CM, Chang IF, Lin SJ, Chou CH (2004a) Effects of three allelopathic phenolics on chlorophyll accumulation of rice (Oryza sativa) seedlings II. Stimulation of Consumption-orientation. Bot Bull Acad Sinica 45:119–125
Yang LT, Mickelson S, See D, Blake TK, Fischer AM (2004b) Genetic analysis of the function of major leaf proteases in barley (Hordeum vulgare L.) nitrogen remobilization. J Exp Bot 55:2607–2616
Zhang SZ, Li YH, Kong CH, Xu XH (2016) Interference of allelopathic wheat with different weeds. Pest Manag Sci 72:172–178
Zuo YQ, Ma S (2007) Allelopathy variation in dryland winter wheat (Triticum aestivum L.) accessions grown on the Loess Plateau of China for about fifty years. Genetic Res Crop Evol 54:1381–1393
Zuo SP, Ma YQ, Deng XP, Li XW (2005) Allelopathy in wheat genotypes during the germination and seedling stages. Allelopathy J 15:21–30
Zuo SP, Liu GB, Li M (2012) Genetic basis of allelopathic potential of winter wheat based on the perspective of quantitative trait locus. Field Crops Res 135:67–73
Zuo S, Li X, Ma Y, Yang S (2014) Soil microbes are linked to the allelopathic potential of different wheat genotypes. Plant Soil 378:49–58
Zwain KHY (1996) Allelopathic effects of wheat (Triticum aestivum L.) on some plant species and nitrogen cycle. Ph.D. Thesis, Al-Mustansriyah University, Baghdad, Iraq
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Liliane Ruess.
Rights and permissions
About this article
Cite this article
Aslam, F., Khaliq, A., Matloob, A. et al. Allelopathy in agro-ecosystems: a critical review of wheat allelopathy-concepts and implications. Chemoecology 27, 1–24 (2017). https://doi.org/10.1007/s00049-016-0225-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-016-0225-x