Abstract
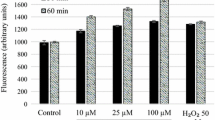
Leishmaniasis is a much-neglected tropical disease, especially in developing countries. Visceral Leishmaniasis (VL) is the most severe form of this infection caused by Leishmania infantum specie. It affects the splenic and hepatic systems and, when left untreated, it is fatal in 95% of cases. There is no human vaccine available and the recommended treatment not only presents adverse effects but it enables the development of resistant strains if interrupted. Hence, the urgent search for new leishmanicidal compounds. In the present work, we evaluated the in vitro leishmanicidal action of new thiosemicarbazone and thiazolidine compounds against the evolutionary forms of L. infantum, as well as their hemolytic activity, ultrastructural alterations, cytotoxicity, and nitric oxide levels on peritoneal macrophages. The inhibitory concentration 50% (IC50) against promastigote forms varied from 8.62 to 34.36 µM. In the evaluation of cytotoxicity, values of CC50 in peritoneal macrophages varied from 31.80 to 196.38 µM. Data showed that compounds JW-16.2 and GT-14 were the most promising in the series as they displayed the highest CC50 (184.58 and 196.38 μM), SI (19 and 15), low hemolytic activity, induced NO production in uninfected and infected macrophages and reduced survival of amastigotes (24.39 and 16.51 μM). Ultrastructural alterations, such as shrinkage of the cell body, shortening of the flagellum, and vacuolization of the cytoplasm, were identified. Promastigote forms treated with compounds GT-14 showed depolarization of mitochondria. Therefore, both compounds are promising due to low cytotoxicity to mammalian cells and leishmanicidal action.








Similar content being viewed by others
References
Aliança A et al. (2017) In vitro evaluation of cytotoxicity and leishmanicidal activity of phthalimido-thiazole derivatives. Eur J Pharm Sci 105:1–10
Basmaciyan L et al. (2018) Temporal analysis of the autophagic and apoptotic phenotypes in Leishmania parasites. Micro Cell 5:404–417
Becerra MC et al. (2012) In vitro activity of N-benzenesulfonylbenzotriazole on Trypanosoma cruzi epimastigote and trypomastigote forms. Exp Parasitol 131:57–62
Bharti SK et al. (2010) Synthesis, anti-bacterial and anti-fungal activities of some novel Schiff bases containing 2,4-disubstituted thiazole ring. Eur J Med Chem 45:651–660
Bozdag-Dundar O et al. (2007) Synthesis and antimicrobial activity of some new thiazolyl thiazolidine-2,4-dione derivatives. Bioorg Med Chem 15:6012–6017
Braga MV et al. (2004) Effects of squalene synthase inhibitors on the growth and ultrastructure of Trypanosoma cruzi. Int J Antimicrob Agents 24:72–78
Brasil (2019) Guia de Vigilância em Saúde. Ministério da Saúde, Brasília, https://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_saude_3ed.pdf. Accessed 20 Dec 2019
Britta EA et al. (2014) Cell death and ultrastructural alterations in Leishmania amazonensis caused by new compound 4-nitrobenzaldehyde thiosemicarbazone derived from Slimonene. BMC Microbiol 14:236
Britta EA et al. (2015) 4-Nitrobenzaldehyde thiosemicarbazone: a new compound derived from S-(-)-limonene that induces mitochondrial alterations in epimastigotes and trypomastigotes of Trypanosoma cruzi. Parasitol 142:978–988
Caldas LA et al. (2019) Antileishmanial activity and ultrastructural changes of sesquiterpene lactones isolated from Calea pinnatifida Asteraceae. Bioorg Chem 83:348–353
Caputto ME et al. (2011) Thiosemicarbazones derived from 1-indanones as new anti-Trypanosoma cruzi agents. Bioorg Med Chem 19:6818–6826
Cardoso MVO et al. (2014) 2-Pyridyl thiazoles as novel anti-Trypanosoma cruzi agents: structural design, synthesis and pharmacological evaluation. Eur J Med Chem 86:48–59
Carvalho EB (2007) Efeito da bomba de infusão de soluções sobre o grau de hemólise em concentrado de hemácias. Rev Bras Hematol Hemoter 29:149–152
Costa LB et al. (2016) Compound profiling and 3D-QSAR studies of hydrazone derivatives with activity against intracellular Trypanosoma cruzi. Bioorg Med Chem 24:1608–1618
Da Silva AC et al. (2017) Aryl thiosemicarbazones: In vitro and immunomodulatory activities against L. amazonensis. Exp Parasitol 177:57–65
De Athayde-filho PF et al. (2000) Mesoionic compounds: amphiphilic heterocyclic betaines. synthesis. Synthesis 2000:1565–1568
De Macedo-Silva ST (2011) Antiproliferative, ultrastructural, and physiological effects of amiodarone on promastigote and amastigote forms of Leishmania amazonensis. Mol Biol Int 2011:1–12
De Oliveira Filho GB et al. (2015) Structural design, synthesis and pharmacological evaluation of 4-thiazolidinones against Trypanosoma cruzi. Bioorg Med Chem 23:7478–7486
De Oliveira Filho GB et al. (2017) Structural design, synthesis and pharmacological evaluation of thiazoles against Trypanosoma cruzi. Eur J Med Chem 141:346–361
El-Hani CN et al. (2012) Apoptosis and apoptotic mimicry in Leishmania: an evolutionary perspective. Front Cell Infect Microbiol 2:1–6
Espíndola JWP et al. (2015) Synthesis and structureeactivity relationship study of a new series of antiparasitic aryloxyl thiosemicarbazones inhibiting Trypanosoma cruzi cruzain. Eur J Med Chem 101:818–835
Franzen RG (2000) Recent advances in the preparation of heterocycles on solid support: a review of the literature. J Comb Chem 2:195–214
Garzoni LR et al. (2004) Selective in vitro effects of the farnesyl pyrophosphate synthase inhibitor risedronate on Trypanosoma cruzi. Int J Antimicrob Agents 23:273–285
Ghosh M et al. (2013) Immunomodulatory effects of antileishmanial drugs. J Antimicrob Chem 68:2834–2838
Glisoni RJ et al. (2012) Antiviral activity against the hepatitis C virus (HCV) of 1-indanone thiosemicarbazones and their inclusion complexes with hydroxypropyl-bcyclodextrin. Eur J Pharm Sci 47:596–603
Gomes PM et al. (2016) Phthalimido-thiazoles as building blocks and their effects on the growth and morphology of Trypanosoma cruzi. Eur J Med Chem 111:46–57
Hernandes MZ et al. (2010) Studies toward the structural optimization of novel thiazolylhydrazone-based potent antitrypanosomal agents. Bioorg Med Chem 18:7826–7835
Holzmuller P et al. (2002) Nitric oxide-mediated proteasome-dependent oligonucleosomal DNA fragmentation in Leishmania amazonensis Amastigotes. Infect Immun 70:3727–3735
Joshi J et al. (2014) A comparative evaluation of efficacy of chemotherapy, immunotherapy and immunochemotherapy in visceral leishmaniasis—an experimental study. Parasitol Intern 63:612–620
Kalegari M et al. (2011) Phytochemical constituents and preliminary toxicity evaluation of leaves from Rourea induta Planch. (Connaraceae). Braz J Pharm Sci 47:635–642
Kaushik NK et al. (2013) Biomedical Importance of Indoles. Molecules 18:6620–6662
Kroemer G et al. (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87:99–163
Liesen AP et al. (2008) Métodos de obtenção, reatividade e importância biológica de 4-tiazolidinonas. Quím Nova 31:369–376
Lima-Junior DS et al. (2013) Inflammasome-derived IL-1ß production induces nitric oxide-mediated resistance to Leishmania. Nat Med 19:909–915
Lipinski CA et al. (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 23:3–25
Lüder CG et al. (2010) Impact of protozoan cell death on parasite-host interactions and pathogenesis. Parasit Vectors 3:116
Lukmantara AY et al. (2013) Synthesis and biological evaluation of substituted 2-benzoylpyridine thiosemicarbazones: novel structure-activity relationships underpinning their anti-proliferative and chelation efficacy. Bioorg Med Chem Lett 23:967–974
Melo JOF et al. (2006) Heterocíclicos 1,2,3-triazólicos: histórico, métodos de preparação, aplicações e atividades farmacológicas. Quím Nova 29:569–579
Menna-Barreto RF et al. (2009) Different cell death pathways induced by drugs in Trypanosoma cruzi: an ultrastructural study. Micron 40:157–168
Muylder G et al. (2011) A screen against Leishmania intracellular Amastigotes: comparison to a promastigote screen and identification of a host cell-specific hit. PLOS Negl Trop Dis 5:e1253
Pavan FR et al. (2010) Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: anti-Mycobacterium tuberculosis activity and cytotoxicity. Eur J Med Chem 45:1898–1905
Pinto EG et al. (2014) Potential of 2-hydroxy-3-phenylsulfanylmethyl-[1,4]-naphthoquinones against Leishmania (L.) infantum: biological activity and structureactivity relationships. PLoS ONE 9:e105127
Preté PSC et al. (2011) Multiple stages of detergent-erythrocyte membrane interaction—a spin label study. Biochim. Biophys Acta 1808:164–170
Santiago EDF (2014) Evaluation of the Anti-Schistosoma mansoni Activity of Thiosemicarbazones and Thiazoles. Antimicrob Agents Chem 58:352–363
Sudo RT et al. (2010) Interaction of morphine with a new alpha2-adrenoceptor agonist in mice. J Pain 11:71–78
Sundar S, Chakravarty J (2015) Investigational drugs for visceral leishmaniasis. Expert Opin Investig Drugs 24:43–59
Verma A, Saraf SK (2008) 4-Thiazolidinone and a biologically active scaffold. Eur J Med Chem 43:897–905
World and Health Organization (WHO) (2020) Leishmaniasis Fact Sheet, http://www.who.int/en/news-room/fact-sheets/detail/leishmaniasis, Accessed 10 May 2020
Yamamoto ES et al. (2015) The effect of ursolic acid on Leishmania (Leishmania) amazonensis is related to programmed cell death and presents therapeutic potential in experimental cutaneous leishmaniasis. PLoS ONE 10:e0144946
Acknowledgements
The authors would like to acknowledge Gabriel Gazzoni and Jana Sandes for the scientific and operational support provided. This work has been carried out with the support of the Co-ordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES) and Oswaldo Cruz Foundation (Fiocruz-Pernambuco)—Brazil.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) and PROEP FIOCRUZ/CNPq, process number 400629/2019-4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Queiroz, C.M., de Oliveira Filho, G.B., Espíndola, J.W.P. et al. Thiosemicarbazone and thiazole: in vitro evaluation of leishmanicidal and ultrastructural activity on Leishmania infantum. Med Chem Res 29, 2050–2065 (2020). https://doi.org/10.1007/s00044-020-02619-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02619-z