Abstract
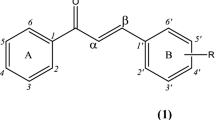
As a part of our program to generate some novel flavonoid frameworks substituted with higher alkyl groups as possible antimicrobial agents, we have in total synthesized twelve novel chalcones (11–16) and their corresponding flavanones (17–22) substituted with either nonyl or dodecyl chains in ring B in very good to excellent yields. The synthesized compounds have been screened for their antimicrobial potential against six bacterial and four fungal strains. The tested compounds, in general, showed significant antibacterial and comparable antifungal activities. While the chalcone (16) with a dodecyl chain showed highly promising antibacterial activity against almost all the organisms tested, the chalcone (13) with nonyl chain showed promising antifungal activity against Candida rugosa and Aspergillus niger strains.



Similar content being viewed by others
References
Blazevic N, Kolbah D, Belin B, Sunjic V, Kajfez F (1979) Hexamethylenetetramine, a versatile reagent in organic synthesis. Synthesis 3:161–176
Buer CS, Imin N, Djordjevic MA (2010) Flavonoids : new roles for old molecules. J Int Plant Biol 52:98–111
Cheng CY, Huges CA, Barnard KR, Larcombe K (2000) Manganese in copper solvent extraction and electrowinning. Hydrometallurgy 58:135–150
Clinical And Laboratory Standards Institute (2008) Performance Standards for Antimicrobial Susceptibility Tests. Eighteen informational supplement M100-S18
Cushnie TPT, Lamb AJ (2005) Antimicrobial activity of flavonoids. Int J Antimicrob Agents 26:343–346
Cushnie TPT, Lamb AJ (2011) Recent advances in understanding the antibacterial properties of flavonoids. Int J Antimicrob Agents 38:99–107
Fowler ZL, Shah K, Panepinto JC, Jacobs A, Koffas MAG (2011) Development of non-natural flavanones as antimicrobial agents. PLoS One 6:1–5
Gordon RJ, Campbell J, Henderson DK, Henry DCR, Swart RM, Tasker PA, White FJ, Wood JL, Yellowlees LJ (2008) Polyacidic multiloading metal extractants. Chem Commun 4801–4803
Haraguchi H, Tanimoto K, Tamura Y, Mizutani K, Kinoshita T (1998) Mode of antibacterial action of retrochalcones from Glycyrrhiza inflata. Phytochemistry 48:125–129
Hwang D, Hyun J, Jo G, Koh D, Lim Y (2011) Synthesis and complete assignment of NMR data of 20 chalcones. Magn Reson Chem 49:41–45
Kromann H, Larsen M, Boesen T, Schonning K, Nielsen SF (2004) Synthesis of prenylated benzaldehydes and their use in the synthesis of analogues of licochalcone A. Eur J Med Chem 39:993–1000
Linday ME (1962) Practical introduction to microbiology. E and F.N. Spon Ltd., London 177
Mandge S, Singh HP, Gupta SD, Moorthy NSHN (2007) Synthesis and characterisation of some chalcone derivatives. Tren Appl Sci Res 2:5256
Marais JPJ, Deavours B, Dixon RA, Ferreira D (2006) In: Grotewold E (ed) The science of flavonoids. Springer, New York, pp 1–46
Nowakowska Z (2007) A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem 42:125–137
Pouramini Z, Moradi A (2012) Comparing of 5-nonylsalicylaldoxime and salicylaldehyde characterisation using magnesium salt formylation process. J Korean Chem Soc 56:357–362
Sperline RP, Song Y, Ma E, Freiser H (1998) Organic constituents of cruds in Cu solvent extraction circuits. II. Photochemical and acid hydrolytic reactions of alkaryl hydroxyoxime reagents. Hydrometallurgy 50:23–38
Stapleton PD, Shah S, Hamilton-Miller JMT, Hara Y, Nagaoka Y, Kumagai A, Uesato S, Taylor PW (2004) Anti-staphylococcus aureus activity and oxaxillin resistance modulating capacity of 3-O-acyl-catechins. Int J Antimicrob Agents 24:374–380
Yoon H, Eom S, Hyun J, Jo G, Hwang D, Lee S, Yong Y, Park JC, Lee YH, Lim Y (2011) 1H and 13C NMR data on hydroxy/methoxy flavonoids and the effects of substituents on chemical shifts. Bull Korean Chem Soc 32:2101–2104
Acknowledgments
We are highly thankful to the Director, CSIR-IICT, Hyderabad, India for support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mallavadhani, U.V., Sahoo, L., Kumar, K.P. et al. Synthesis and antimicrobial screening of some novel chalcones and flavanones substituted with higher alkyl chains. Med Chem Res 23, 2900–2908 (2014). https://doi.org/10.1007/s00044-013-0876-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0876-x