Abstract
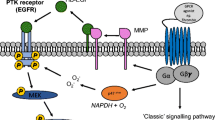
G protein-coupled receptor (GPCR) signalling is mediated through transactivation-independent signalling pathways or the transactivation of protein tyrosine kinase receptors and the recently reported activation of the serine/threonine kinase receptors, most notably the transforming growth factor-β receptor family. Since the original observation of GPCR transactivation of protein tyrosine kinase receptors, there has been considerable work on the mechanism of transactivation and several pathways are prominent. These pathways include the “triple membrane bypass” pathway and the generation of reactive oxygen species. The recent recognition of GPCR transactivation of serine/threonine kinase receptors enormously broadens the GPCR signalling paradigm. It may be predicted that the transactivation of serine/threonine kinase receptors would have mechanistic similarities with transactivation of tyrosine kinase pathways; however, initial studies suggest that these two transactivation pathways are mechanistically distinct. Important questions are the relative importance of tyrosine and serine/threonine transactivation pathways, the contribution of transactivation to overall GPCR signalling, mechanisms of transactivation and the range of cell types in which this phenomenon occurs. The ultimate significance of transactivation-dependent signalling remains to be defined but it appears to be prominent and if so will represent a new cell signalling frontier.

Similar content being viewed by others
References
Marinissen MJ, Gutkind JS (2001) G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci 22(7):368–376
Pierce KL, Premont RT, Lefkowitz RJ (2002) Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3(9):639–650. doi:10.1038/nrm908
Spehr M, Munger SD (2009) Olfactory receptors: G protein-coupled receptors and beyond. J Neurochem 109(6):1570–1583. doi:10.1111/j.1471-4159.2009.06085.x
Robison GA, Butcher RW, Sutherland EW (1967) Adenyl cyclase as an adrenergic receptor. Ann N Y Acad Sci 139(3):703–723. doi:10.1111/j.1749-6632.1967.tb41239.x
Gilman AG (1995) Nobel Lecture. G proteins and regulation of adenylyl cyclase. Biosci Rep 15(2):65–97
Rodbell M (1995) Nobel Lecture. Signal transduction: evolution of an idea. Biosci Rep 15(3):117–133
Kobilka BK (2007) G protein coupled receptor structure and activation. Biochim Biophys Acta 1768(4):794–807. doi:10.1016/j.bbamem.2006.10.021
Lefkowitz RJ (2004) Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol Sci 25(8):413–422. doi:10.1016/j.tips.2004.06.006
Lefkowitz RJ (2007) Seven transmembrane receptors: something old, something new. Acta Physiol 190(1):9–19. doi:10.1111/j.1365-201X.2007.01693.x
Chung KY, Rasmussen SG, Liu T, Li S, DeVree BT, Chae PS, Calinski D, Kobilka BK, Woods VL Jr, Sunahara RK (2011) Conformational changes in the G protein Gs induced by the beta2 adrenergic receptor. Nature 477(7366):611–615. doi:10.1038/nature10488
Gether U, Lin S, Ghanouni P, Ballesteros JA, Weinstein H, Kobilka BK (1997) Agonists induce conformational changes in transmembrane domains III and VI of the beta2 adrenoceptor. EMBO J 16(22):6737–6747. doi:10.1093/emboj/16.22.6737
Neer EJ (1995) Heterotrimeric G proteins: organizers of transmembrane signals. Cell 80(2):249–257
Rosenbaum DM, Rasmussen SG, Kobilka BK (2009) The structure and function of G-protein-coupled receptors. Nature 459(7245):356–363. doi:10.1038/nature08144
Vogel R, Siebert F (2001) Conformations of the active and inactive states of opsin. J Biol Chem 276(42):38487–38493. doi:10.1074/jbc.M105423200
Black JW, Stephenson JS (1962) Pharmacology of a new adrenergic beta-receptor-blocking compound (Nethalide). Lancet 2(7251):311–314
Black JW, Duncan WA, Durant CJ, Ganellin CR, Parsons EM (1972) Definition and antagonism of histamine H 2-receptors. Nature 236(5347):385–390
Gutkind JS (1998) Cell growth control by G protein-coupled receptors: from signal transduction to signal integration. Oncogene 17(11):1331–1342. doi:10.1038/sj.onc.1202186
Luttrell LM (2008) Reviews in molecular biology and biotechnology: transmembrane signaling by G protein-coupled receptors. Mol Biotechnol 39(3):239–264. doi:10.1007/s12033-008-9031-1
Katritch V, Cherezov V, Stevens RC (2013) Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol 53:531–556. doi:10.1146/annurev-pharmtox-032112-135923
Daub H, Weiss FU, Wallasch C, Ullrich A (1996) Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature 379(6565):557–560
Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A (1999) EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402(6764):884–888. doi:10.1038/47260
Kamato D, Burch ML, Piva TJ, Rezaei HB, Rostam MA, Xu S, Zheng W, Little PJ, Osman N (2013) Transforming growth factor-beta signalling: role and consequences of Smad linker region phosphorylation. Cell Signal 25(10):2017–2024. doi:10.1016/j.cellsig.2013.06.001
Rezaei HB, Kamato D, Ansari G, Osman N, Little PJ (2011) Cell biology of Smad2/3 linker region phosphorylation in vascular smooth muscle. Clin Exp Pharmacol Physiol. doi:10.1111/j.1440-1681.2011.05592.x
Burch ML, Yang SN, Ballinger ML, Getachew R, Osman N, Little PJ (2010) TGF-β stimulates biglycan synthesis via p38 and ERK phosphorylation of the linker region of Smad 2. Cell Mol Life Sci 67:2077–2090
Burch ML, Zheng W, Little PJ (2011) Smad linker region phosphorylation in the regulation of extracellular matrix synthesis. Cell Mol Life Sci 68(1):97–107. doi:10.1007/s00018-010-0514-4
Kamato D, Burch ML, Osman N, Zheng W, Little PJ (2013) Therapeutic implications of endothelin and thrombin G-protein-coupled receptor transactivation of tyrosine and serine/threonine kinase cell surface receptors. J Pharm Pharmacol 65(4):465–473. doi:10.1111/j.2042-7158.2012.01577.x
Little PJ, Burch ML, Al-Aryahi S, Zheng W (2011) The paradigm of g protein receptor transactivation: a mechanistic definition and novel example. Sci World J 11:709–714. doi:10.1100/tsw.2011.75
Wynne BM, Chiao CW, Webb RC (2009) Vascular smooth muscle cell signaling mechanisms for contraction to angiotensin II and endothelin-1. J Am Soc Hypertension 3(2):84–95. doi:10.1016/j.jash.2008.09.002
Murasawa S, Mori Y, Nozawa Y, Gotoh N, Shibuya M, Masaki H, Maruyama K, Tsutsumi Y, Moriguchi Y, Shibazaki Y, Tanaka Y, Iwasaka T, Inada M, Matsubara H (1998) Angiotensin II type 1 receptor-induced extracellular signal-regulated protein kinase activation is mediated by Ca2+/calmodulin-dependent transactivation of epidermal growth factor receptor. Circ Res 82(12):1338–1348
Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F (2005) Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med 11(8):867–874. doi:10.1038/nm1275
Thomas WG, Brandenburger Y, Autelitano DJ, Pham T, Qian H, Hannan RD (2002) Adenoviral-directed expression of the type 1A angiotensin receptor promotes cardiomyocyte hypertrophy via transactivation of the epidermal growth factor receptor. Circ Res 90(2):135–142
George AJ, Thomas WG, Hannan RD (2010) The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer 10(11):745–759. doi:10.1038/nrc2945
Burch ML, Ballinger ML, Yang SN, Getachew R, Itman C, Loveland K, Osman N, Little PJ (2010) Thrombin stimulation of proteoglycan synthesis in vascular smooth muscle is mediated by protease-activated receptor-1 transactivation of the transforming growth factor beta type I receptor. J Biol Chem 285(35):26798–26805. doi:10.1074/jbc.M109.092767
Little PJ, Burch ML, Getachew R, Al-aryahi S, Osman N (2010) Endothelin-1 stimulation of proteoglycan synthesis in vascular smooth muscle is mediated by endothelin receptor transactivation of the transforming growth factor-[beta] type I receptor. J Cardiovasc Pharmacol 56(4):360–368. doi:10.1097/FJC.0b013e3181ee6811
Williams KJ, Tabas I (1995) The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 15(5):551–561. doi:10.1161/01.atv.15.5.551
Little PJ, Tannock L, Olin KL, Chait A, Wight TN (2002) Proteoglycans synthesized by arterial smooth muscle cells in the presence of transforming growth factor-β1 exhibit increased binding to LDLs. Arterioscler Thromb Vasc Biol 22(1):55–60. doi:10.1161/hq0102.101100
Yang SNY, Burch ML, Tannock LR, Evanko S, Osman N, Little PJ (2010) Transforming growth factor-β regulation of proteoglycan synthesis in vascular smooth muscle: contribution to lipid binding and accelerated atherosclerosis in diabetes. J of Diabetes 2(4):233–242. doi:10.1111/j.1753-0407.2010.00089.x
Burch ML, Getachew R, Osman N, Febbraio MA, Little PJ (2013) Thrombin-mediated proteoglycan synthesis utilizes both protein-tyrosine kinase and serine/threonine kinase receptor transactivation in vascular smooth muscle cells. J Biol Chem 288(10):7410–7419. doi:10.1074/jbc.M112.400259
Sorescu D (2006) Smad3 mediates angiotensin II- and TGF-beta1-induced vascular fibrosis: Smad3 thickens the plot. Circ Res 98(8):988–989. doi:10.1161/01.RES.0000221824.87718.c0
Leask A (2010) Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res 106(11):1675–1680. doi:10.1161/CIRCRESAHA.110.217737
Xu MY, Porte J, Knox AJ, Weinreb PH, Maher TM, Violette SM, McAnulty RJ, Sheppard D, Jenkins G (2009) Lysophosphatidic acid induces alphavbeta6 integrin-mediated TGF-beta activation via the LPA2 receptor and the small G protein G alpha(q). Am J Pathol 174(4):1264–1279. doi:10.2353/ajpath.2009.080160
Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D (2006) Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J Clin Invest 116(6):1606–1614. doi:10.1172/JCI27183
Berry C, Touyz R, Dominiczak AF, Webb RC, Johns DG (2001) Angiotensin receptors: signaling, vascular pathophysiology, and interactions with ceramide. Am J Physiol Heart Circ Physiol 281(6):H2337–H2365
Wetzker R, Bohmer FD (2003) Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol 4(8):651–657. doi:10.1038/nrm1173
Daub H, Wallasch C, Lankenau A, Herrlich A, Ullrich A (1997) Signal characteristics of G protein-transactivated EGF receptor. EMBO J 16(23):7032–7044. doi:10.1093/emboj/16.23.7032
Voisin L, Foisy S, Giasson E, Lambert C, Moreau P, Meloche S (2002) EGF receptor transactivation is obligatory for protein synthesis stimulation by G protein-coupled receptors. Am J Physiol Cell Physiol 283(2):C446–C455. doi:10.1152/ajpcell.00261.2001
Gschwind A, Hart S, Fischer OM, Ullrich A (2003) TACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cells. EMBO J 22(10):2411–2421. doi:10.1093/emboj/cdg231
Frank GD, Eguchi S (2003) Activation of tyrosine kinases by reactive oxygen species in vascular smooth muscle cells: significance and involvement of EGF receptor transactivation by angiotensin II. Antioxid Redox Signal 5(6):771–780. doi:10.1089/152308603770380070
Shida D, Kitayama J, Yamaguchi H, Yamashita H, Mori K, Watanabe T, Yatomi Y, Nagawa H (2004) Sphingosine 1-phosphate transactivates c-Met as well as epidermal growth factor receptor (EGFR) in human gastric cancer cells. FEBS Lett 577(3):333–338. doi:10.1016/j.febslet.2004.10.024
Kalmes A, Daum G, Clowes AW (2001) EGFR transactivation in the regulation of SMC function. Ann N Y Acad Sci 947:42–54 discussion 54-45
Liu Y, Li M, Warburton RR, Hill NS, Fanburg BL (2007) The 5-HT transporter transactivates the PDGFbeta receptor in pulmonary artery smooth muscle cells. FASEB J 21(11):2725–2734. doi:10.1096/fj.06-8058com
Puehringer D, Orel N, Luningschror P, Subramanian N, Herrmann T, Chao MV, Sendtner M (2013) EGF transactivation of Trk receptors regulates the migration of newborn cortical neurons. Nat Neurosci 16(4):407–415. doi:10.1038/nn.3333
Yokote H, Fujita K, Jing X, Sawada T, Liang S, Yao L, Yan X, Zhang Y, Schlessinger J, Sakaguchi K (2005) Trans-activation of EphA4 and FGF receptors mediated by direct interactions between their cytoplasmic domains. Proc Natl Acad Sci USA 102(52):18866–18871. doi:10.1073/pnas.0509741102
Zhao D, Bakirtzi K, Zhan Y, Zeng H, Koon HW, Pothoulakis C (2011) Insulin-like growth factor-1 receptor transactivation modulates the inflammatory and proliferative responses of neurotensin in human colonic epithelial cells. J Biol Chem 286(8):6092–6099. doi:10.1074/jbc.M110.192534
Wang HH, Hsieh HL, Yang CM (2010) Calmodulin kinase II-dependent transactivation of PDGF receptors mediates astrocytic MMP-9 expression and cell motility induced by lipoteichoic acid. J Neuroinflamm 7:84. doi:10.1186/1742-2094-7-84
Tsai CL, Chen WC, Lee IT, Chi PL, Cheng SE, Yang CM (2014) c-Src-dependent transactivation of PDGFR contributes to TNF-alpha-induced MMP-9 expression and functional impairment in osteoblasts. Bone 60:186–197. doi:10.1016/j.bone.2013.12.014
Linseman DA, Benjamin CW, Jones DA (1995) Convergence of angiotensin II and platelet-derived growth factor receptor signaling cascades in vascular smooth muscle cells. J Biol Chem 270(21):12563–12568
Heeneman S, Haendeler J, Saito Y, Ishida M, Berk BC (2000) Angiotensin II induces transactivation of two different populations of the platelet-derived growth factor beta receptor. Key role for the p66 adaptor protein Shc. J Biol Chem 275(21):15926–15932. doi:10.1074/jbc.M909616199
Mondorf UF, Geiger H, Herrero M, Zeuzem S, Piiper A (2000) Involvement of the platelet-derived growth factor receptor in angiotensin II-induced activation of extracellular regulated kinases 1 and 2 in human mesangial cells. FEBS Lett 472(1):129–132
Goppelt-Struebe M, Fickel S, Reiser CO (2000) The platelet-derived-growth-factor receptor, not the epidermal-growth-factor receptor, is used by lysophosphatidic acid to activate p42/44 mitogen-activated protein kinase and to induce prostaglandin G/H synthase-2 in mesangial cells. Biochem J 345(Pt 2):217–224
Herrlich A, Daub H, Knebel A, Herrlich P, Ullrich A, Schultz G, Gudermann T (1998) Ligand-independent activation of platelet-derived growth factor receptor is a necessary intermediate in lysophosphatidic, acid-stimulated mitogenic activity in L cells. Proc Natl Acad Sci USA 95(15):8985–8990
George AJ, Purdue BW, Gould CM, Thomas DW, Handoko Y, Qian H, Quaife-Ryan GA, Morgan KA, Simpson KJ, Thomas WG, Hannan RD (2013) A functional siRNA screen identifies genes modulating angiotensin II-mediated EGFR transactivation. J Cell Sci 126(Pt 23):5377–5390. doi:10.1242/jcs.128280
Couet J, Sargiacomo M, Lisanti MP (1997) Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem 272(48):30429–30438
Hua H, Munk S, Whiteside CI (2003) Endothelin-1 activates mesangial cell ERK1/2 via EGF-receptor transactivation and caveolin-1 interaction. Am J Physiol Renal Physiol 284(2):F303–F312. doi:10.1152/ajprenal.00127.2002
Ushio-Fukai M, Hilenski L, Santanam N, Becker PL, Ma Y, Griendling KK, Alexander RW (2001) Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J Biol Chem 276(51):48269–48275. doi:10.1074/jbc.M105901200
Blume-Jensen P, Hunter T (2001) Oncogenic kinase signalling. Nature 411(6835):355–365
Burch ML, Osman N, Getachew R, Al-Aryahi S, Poronnik P, Zheng W, Hill MA, Little PJ (2012) G protein coupled receptor transactivation: extending the paradigm to include serine/threonine kinase receptors. Int J Biochem Cell Biol 44(5):722–727. doi:10.1016/j.biocel.2012.01.018
Chung H, Ramachandran R, Hollenberg MD, Muruve DA (2013) Proteinase-activated receptor-2 transactivation of epidermal growth factor receptor and transforming growth factor-beta receptor signaling pathways contributes to renal fibrosis. J Biol Chem 288(52):37319–37331. doi:10.1074/jbc.M113.492793
Ruoslahti E, Pierschbacher MD (1987) New perspectives in cell adhesion: RGD and integrins. Science 238(4826):491–497
Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110(6):673–687
Tatler AL, John AE, Jolly L, Habgood A, Porte J, Brightling C, Knox AJ, Pang L, Sheppard D, Huang X, Jenkins G (2011) Integrin alphavbeta5-mediated TGF-beta activation by airway smooth muscle cells in asthma. J Immunol 187(11):6094–6107. doi:10.4049/jimmunol.1003507
Scotton CJ, Krupiczojc MA, Konigshoff M, Mercer PF, Lee YC, Kaminski N, Morser J, Post JM, Maher TM, Nicholson AG, Moffatt JD, Laurent GJ, Derian CK, Eickelberg O, Chambers RC (2009) Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J Clin Investig 119(9):2550–2563. doi:10.1172/JCI33288
Worthington JJ, Czajkowska BI, Melton AC, Travis MA (2011) Intestinal dendritic cells specialize to activate transforming growth factor-beta and induce Foxp3+ regulatory T cells via integrin alphavbeta8. Gastroenterology 141(5):1802–1812. doi:10.1053/j.gastro.2011.06.057
Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA (1996) Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell 85(5):683–693
Kramarenko II, Bunni MA, Raymond JR, Garnovskaya MN (2010) Bradykinin B2 receptor interacts with integrin alpha5beta1 to transactivate epidermal growth factor receptor in kidney cells. Mol Pharmacol 78(1):126–134. doi:10.1124/mol.110.064840
Belmadani S, Zerfaoui M, Boulares HA, Palen DI, Matrougui K (2008) Microvessel vascular smooth muscle cells contribute to collagen type I deposition through ERK1/2 MAP kinase, alphavbeta3-integrin, and TGF-beta1 in response to ANG II and high glucose. Am J Physiol Heart Circ Physiol 295(1):H69–H76. doi:10.1152/ajpheart.00341.2008
Koch WJ, Hawes BE, Allen LF, Lefkowitz RJ (1994) Direct evidence that Gi-coupled receptor stimulation of mitogen-activated protein kinase is mediated by G beta gamma activation of p21ras. Proc Natl Acad Sci U S A 91(26):12706–12710
Little PJ (2013) GPCR responses in vascular smooth muscle can occur predominantly through dual transactivation of kinase receptors and not classical Galphaq protein signalling pathways. Life Sci 92(20–21):951–956. doi:10.1016/j.lfs.2013.03.017
Jain R, Shaul PW, Borok Z, Willis BC (2007) Endothelin-1 induces aveolar epithelial mesenchymal transition through endothelin type A receptor mediated production of TGF-beta 1. Am J Respir Cell Mol Biol 37(1):38–47
Yu B, Zhao X, Yang C, Crane J, Xian L, Lu W, Wan M, Cao X (2012) Parathyroid hormone induces differentiation of mesenchymal stromol/stem cells by enhancing bone morphogenetic protein signaling. J Bone Miner Res 27(9):2001–2014
Acknowledgments
This study was supported by a National Heart Foundation of Australia grant-in-aid (PJL/NO). MAR is supported by the Academic Trainee Scholarship from the International Islamic University of Malaysia. We thank the Ministry of Foreign Experts of the Government of the People’s Republic of China for support by way of High End Professor (Education) Award through Zhongshan (Sun Yat-sen) University (PJL).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamato, D., Rostam, M.A., Bernard, R. et al. The expansion of GPCR transactivation-dependent signalling to include serine/threonine kinase receptors represents a new cell signalling frontier. Cell. Mol. Life Sci. 72, 799–808 (2015). https://doi.org/10.1007/s00018-014-1775-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1775-0