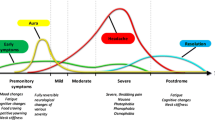
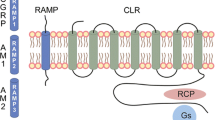
Abstract
Migraine is a complex and still debatable neurovascular disorder that is on the rise globally, affecting patients of all ages. Various drugs are available for acute and chronic management, but migraine appears to be a chronic and progressive condition, with 5.1% of patients ultimately becoming refractory to drugs. For the acute and preventative management of migraine, newer molecules targeting calcitonin gene-related peptide (CGRP) have been approved and others are in various phases of development. This narrative review aims to give an overview of migraine therapy with special emphasis on CGRP and the molecules targeting this that are either approved or have completed phase III trials.
Similar content being viewed by others
References
Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalgia. 2018;38(1):1–211.
Goadsby PJ. Headache. Harrison’s principles of internal medicine. 20th ed. New York: McGrawhill Education; 2018. p. 85–9.
Steiner TJ, Stovner LJ, Birbeck GL. Migraine: the seventh disabler. J Headache Pain. 2013;14(1):1.
Ray BK, Paul N, Hazra A, et al. Prevalence, burden, and risk factors of migraine: a community-based study from Eastern India. Neurol India. 2017;65:1280–8.
Steiner TJ, Stovner LJ, Vos T. GBD 2015: migraine is the third cause of disability in under 50s. J Headache Pain. 2016;17(1):104.
Part I: The primary headaches. International headache society classification ICHD-3. https://ichd-3.org. Accessed 11 July 2019.
May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol. 2016;12(8):455–64.
Lipton RB. Tracing transformation: chronic migraine classification, progression, and epidemiology. Neurology. 2009;72:S3–7.
Schwedt TJ. Chronic migraine. BMJ. 2014;348:g1416.
Park JW, Chu MK, Kim JM, et al. Analysis of trigger factors in episodic migraineurs using a smartphone headache diary applications. PLoS One. 2016;11(2):e0149577.
Hoffmann J, Recober A. Migraine and triggers: post hoc ergo propter hoc? Curr Pain Headache Rep. 2013;17(10):370.
Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol. 2017;16(1):76–87.
Goadsby PJ. Pathophysiology of migraine. Ann Indian Acad Neurol. 2012;15:S15–22.
Cutrer FM. Pathophysiology of migraine. Semin Neurol. 2010;30(2):120–30.
Rahmann A, Wienecke T, Hansen JM, et al. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28:226–36.
Amin FM, Asghar MS, Hougaard A, et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. Lancet Neurol. 2013;12:454–61.
Edvinsson L, Villalon CM, MaassenVanDenBrink A. Basic mechanisms of migraine and its acute treatment. Pharmacol Ther. 2012;136:319–33.
Bigley GK. Headache. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston: Elsevier; 1990.
Drake R, Vogl W, Mitchell A. Gray’s anatomy for students. 2nd ed. Philadelphia: Elsevier; 2005.
Fricke B, Andres KH, Von During M. Nerve fibers innervating the cranial and spinal meninges: morphology of nerve fiber terminals and their structural integration. Microsc Res Tech. 2001;53:96–105.
Mayberg M, Langer RS, Zervas NT, et al. Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science. 1981;213:228–30.
Perini F, Dandrea G, Galloni E, et al. Plasma cytokine levels in migraineurs and controls. Headache. 2005;45:926–31.
Levy D. Migraine pain, meningeal inflammation, and mast cells. Curr Pain Headache Rep. 2009;13:237–40.
Levy D, Burstein R, Kainz V, et al. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130:166–76.
Rosenfeld MG, Mermod JJ, Amara SG, et al. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983;304:129–35.
van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev. 1997;21:649–78.
Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med. 2011;13:e36.
Russo AF. Calcitonin gene related peptide (CGRP) a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533–52.
Amara SG, Arriza JL, Leff SE, et al. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science. 1985;229:1094–7.
Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–34.
Mulderry PK, Ghatei MA, Spokes RA, et al. Differential expression of α- CGRP and β-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience. 1988;25:195–205.
Tippins JR. CGRP: a novel neuropeptide from the calcitonin gene is the most potent vasodilator known. J Hypertens. 1986;4(5):S102–5.
Lassen LH, Jacobsen VB, Haderslev PA, et al. Involvement of calcitonin gene-related peptide in migraine: regional cerebral blood flow and blood flow velocity in migraine patients. J Headache Pain. 2008;9:151–7.
Wellman GC, Quayle JM, Standen NB. ATP-sensitive K¤ channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. J Physiol. 1998;507(1):117–29.
Levy D, Burstein R. The vascular theory of migraine: leave it or love it? Ann Neurol. 2011;69:600–1.
Goadsby PJ. The vascular theory of migraine: a great story wrecked by the facts. Brain. 2009;132:6–7.
Asghar MS, Hansen AE, Amin FM, et al. Evidence for a vascular factor in migraine. Ann Neurol. 2011;69:635–45.
Zhang Z, Winborn CS, Prado B, et al. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci. 2007;27:2693–703.
Ottosson A, Edvinsson L. Release of histamine from dural mast cells by substance P and calcitonin gene-related peptide. Cephalalgia. 1997;17:166–74.
Capuano A, De Corato A, Lisi L, et al. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: relevance for migraine pathology. Mol Pain. 2009;5:43.
Seybold VS. The role of peptides in central sensitization. Handb Exp Pharmacol. 2009;194:451–91.
Anthony M, Hinterberger H, Lance JW. Plasma serotonin in migraine and stress. Arch Neurol. 1967;16:544–52.
Kimball RW, Friedman AP, Vallejo E. Effect of serotonin in migraine patients. Neurology. 1960;10:107–11.
Jacon SN, Nienborg H. Monoaminergic neuromodulation of sensory processing. Front Neural Circuits. 2018;12:51.
Berman NEJ, Puri V, Chandrala S, et al. Serotonin in trigeminal ganglia of female rodents: relevance to menstrual migraine. Headache. 2006;46:1230–4.
Brewerton TD, Murphy DL, Meuller EA, et al. Induction of migraine-like headaches by the serotonin agonist, m-chlorophenylpiperazine. Clin Pharmacol Ther. 1988;43:605–8.
Ferrari MD, Melamed E, Gawel MJ. Treatment of migraine attacks with sumatriptan. N Engl J Med. 1991;325:316–8.
Goadsby PJ. Migraine and other primary headache disorder. Harrison’s Principles of Internal Medicine. 20th ed. New York: McGrawhill Education; 2018. p. 3096–108.
Negm AA, Furst DE. Nonsteroidal anti-inflammatory drugs, disease modifying antirheumatic drugs, nonopioid analgesics and drugs used in gout. Basic and clinical pharmacology. 14th ed. New York: Mcgrawhill Education; 2018. p. 642–66.
Kanniainen HH. Treatment of acute migraine attack: ibuprofen and placebo compared. Headache. 1989;29(8):507–9.
Myllya VV, Havanka H, Herrala L, et al. Tolfenamic acid rapid release versus sumatriptan in the acute treatment of migraine: comparable effect in a double-blind, randomized, controlled, parallel-group study. Headache. 1998;38(3):201–7.
Nestvold K, Kloster R, Partinen M, Sulkava R. Treatment of acute migraine attack: naproxen and placebo compared. Cephalalgia. 1985;5(2):115–9.
Dahlöf C, Björkman R. Diclofenac-K (50 and 100 mg) and placebo in the acute treatment of migraine. Cephalalgia. 1993;13(2):117–23.
Pardutz A, Schoenen J. NSAIDs in the acute treatment of migraine: a review of clinical and experimental data. Pharmaceuticals. 2010;3:1966–87.
Wooltorton E. Risk of stroke, gangrene from ergot drug interactions. CMAJ. 2003;168(8):1015.
Anderson JR, Drehsen G, Pitman IH. Effect of caffeine on ergotamine absorption from rat small intestine. J Pharm Sci. 1981;70(6):651–7.
Sibley DR, Hazelwood LA, Amara SG. 5-Hydroxytryptamine (serotonin) and dopamine. Goodman and Gilman’s pharmacological basis of therapeutics. 13th ed. New York: McGrawhill Education; 2018. p. 225–42.
Ahn AH, Basbaum AI. Where do triptans act in treatment of migraine? Pain. 2005;115(1–2):1–4.
Moskowitz MA, Cutrer FM. Sumatriptan: a receptor-targeted treatment for migraine. Annu Rev Med. 1993;44:145–54.
Ferrari MD, Roon KI, Lipton RB, et al. Oral triptans (serotonin 5-HT1B/1D agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet. 2001;358(9294):1668–75.
Volans GN. Migraine and drug absorption. Clin Pharmacokinet. 1978;3(4):313–8.
BET 1. Metoclopramide or prochlorperazine for headache in acute migraine? Emerg Med J. 2013;30:595–6.
Lipton RB, Baggish JS, Stewart WF, et al. Efficacy and safety of acetaminophen in the treatment of migraine. Arch Intern Med. 2000;160(22):3486–92.
Pfaffenrath V, Rehm M. Migraine in pregnancy: what are the safest treatment options? Drug Saf. 1998;19(5):383–8.
Friedman BW, Kapoor A, Friedman MS, et al. The relative efficacy of meperidine for the treatment of acute migraine: a meta-analysis of randomized controlled trials. Ann Emerg Med. 2008;52(6):705–13.
Koella WP. CNS-related (side-)effects of beta-blockers with special reference to mechanisms of action. Eur J Clin Pharmacol. 1985;28:55–63.
Gelmers HJ. Calcium-channel blockers in the treatment of migraine. Am J Cardiol. 1985;55(3):139B–43B.
Mansoureh T, Jird MR, Nilavari K, et al. Cinnarizine in refractory migraine prophylaxis: efficacy and tolerability: a comparison with sodium valproate. J Headache Pain. 2008;9(2):77–82.
Schmidt R, Oestreich W. Flunarizine in migraine prophylaxis: the clinical experience. J Cardiovasc Pharmacol. 1991;18(Suppl 8):S21–6.
Garza I, Swanson JW. Prophylaxis of migraine. Neuropsychiatr Dis Treat. 2006;2(3):281–91.
Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144–52.
Brandes JL, Saper JR, Diamond M, et al. Topiramate for migraine prevention: a randomized controlled trial. JAMA. 2004;291(8):965–73.
Hering R, Kuritzky A. Sodium valproate in the prophylactic treatment of migraine: a double-blind study versus placebo. Cephalalgia. 1992;12:81–4.
Cutrer FM, Limmroth V. Moskowitz. Possible mechanisms of valproate in migraine prophylaxis. Cephalalgia. 1997;17(2):93–100.
Irimia P, Palma JA, Torron RF, Vila EM. Refractory migraine in a headache clinic population. BMC Neurol. 2011;11:94.
Sheykhzade M, Amandi N, Pla MV, et al. Binding and functional pharmacological characteristics of gepant-type antagonists in rat brain and mesenteric arteries. Vascul Pharmacol. 2017;90:36–43.
Tong G, Savant I, Jariwala N, et al. Phase I single and multiple dose study to evaluate the safety, tolerability and pharmacokinetics of BMS-927711 in healthy subjects [abstract]. J Headache Pain. 2013;14(Suppl 1):118.
Marcus R, Goadsby PJ, Dodick D, et al. BMS-927711 for the acute treatment of migraine: a double blind, randomized, placebo controlled, dose ranging trial. Cephalgia. 2014;34(2):114–25.
Lipton RB, Croop R, Stock EG, et al. Rimegepant, an oral calcitonin gene related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381:142–9.
Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomized, phase 3, double-blind, placebo-controlled trial. Lancet. https://doi.org/10.1016/S0140-6736(19)31606-X.
Lipton RB, Conway CM, Stock EG, et al. Efficacy, safety and tolerability of rimegepant 75 mg, an oral CGRP receptor antagonist, for the acute treatment of migraine: results from a phase 3, double blind, randomized, placebo controlled trial, study 301 [poster]. In: 60th Annual Scientific Meeting of the American Headache Society; 2018. https://www.biohavenpharma.com/sites/default/files/documents/Rimegepant-Phase-3-Study-301_AHS-2018-Late-Breaking-Poster-Presentation-1.pdf. Accessed 25 July 2019.
A phase 2/3 randomized double blind placebo-controlled study to evaluate the efficacy and safety of rimegepant in migraine prevention. https://clinicaltrials.gov/ct2/show/NCT03732638. Accessed 11 July 2019.
A phase 2b multicenter randomized double blind placebo controlled pharmacokinetic Study of MK-1602 in the treatment of Acute Migraine (MK-1602-007). https://clinicaltrials.gov/ct2/show/NCT01657370. Accessed 11 July 2019.
Voss T, Lipton RB, Dodick DW, et al. A phase 2b randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalgia. 2016;36(9):887–98.
Allergan announces positive top line phase 3 clinical trial for ubrogepant: an oral CGRP receptor antagonist for the acute treatment of migraine (February 06, 2018). https://www.allergan.com/news/news/thomson-reuters/allergan-announc-es-positive-phase-3-resul. Accessed 11 July 2019.
Trugman JM, Dodick DW, Ailani J, et al. Efficacy, safety, and tolerability of ubrogepant for the acute treatment of migraine: results from a single-attack phase 3 study, ACHIEVE II [abstract no. S38.008]. Neurology. 2019;92(15 Suppl).
Tfelt-Hansen P, Loder E. The emperor’s new Gepants: are the effects of the new oral CGRP antagonists clinically meaningful? Headache. 2018;59:113–7.
Goadsby PJ, Dodick DW, Trugman JM, et al. Orally administered atogepant was efficacious, safe, and tolerable for the prevention of migraine: results from a phase 2b/3 study [abstract no. S17.001]. Neurology. 2019;92(15 Suppl).
A phase 3 multicentre randomized double blind placebo-controlled parallel group study to evaluate the efficacy, safety and tolerability of oral atogepant for the prevention of migraine in participants with episodic migraine. https://clinicaltrials.gov/ct2/show/NCT03777059. Accessed 11 July 2019.
A phase 3 multicentre randomized open label study to evaluate the long term safety and tolerability of oral atogepant for the prevention of migraine in participants with episodic migraine. https://clinicaltrials.gov/ct2/show/NCT03700320. Accessed 11 July 2019.
Ho TW, Connor KM, Zhang Y, et al. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology. 2014;83(11):958–66.
Monteith D, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of the CGRP binding monoclonal antibody LY2951742 (galcanezumab) in healthy volunteers. Front Pharmacol. 2017;8:740.
Oakes TMM, Skijarevski V, Zhang Q, et al. Safety of galcanezumab in patients with episodic migraine: a randomized placebo-controlled dose-ranging Phase 2b study. Cephalalgia. 2018;38(6):1015–25.
Detke HC, Goadsay PJ, Wang S, et al. Galcanezumab in chronic migraine: the randomized, double blind, placebo controlled REGAIN study. Neurology. 2018;91(24):e2211–21.
Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080–8.
Skljarevski V, Matharu M, Millen BA, et al. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalgia. 2018;38(8):1442–54.
Drug Approval Package: Emgality (galcanezumab-gnlm). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761063Orig1s000TOC.cfm. Accessed 11 July 2019.
Highlights of prescribing information (Galcanezumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf. Accessed 11 July 2019.
A randomized double blind placebo controlled study of galcanezumab in participants 6 to 17 years of age with episodic migraine (REBUILD). https://clinicaltrials.gov/ct2/show/NCT03432286.Accessed 11 July 2019.
A randomized double blind placebo controlled study of galcanezumab in adults with treatment resistant migraine—the CONQUER study. https://clinicaltrials.gov/ct2/show/NCT03559257. Accessed 11 July 2019.
Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine; a randomized clinical trial. JAMA. 2018;319(19):1999–2008.
Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113–22.
Novel drug approvals for 2018 (fremanezumab). https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2018. Accessed 11 July 2019.
Highlights of prescribing information (fremanezumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf. Accessed 11 July 2019.
A study to explore the long-term safety of TEV-48125 (fremanezumab) for the prevention of cluster headache (ENFORCE). https://clinicaltrials.gov/ct2/show/NCT03107052. Accessed 11 July 2019.
Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene related peptide, for the prevention of frequent episodic migraine: a randomized, double blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014;11:1100–7.
Baker B, Hodsman P, Smith J. PK & PD supporting a single dose, placebo-controlled randomized ascending dose study of ALD403, a humanized anti-calcitonin gene-related peptide (CGRP) monoclonal antibody administered IV or SC. https://www.alderbio.com/wp-content/uploads/2014/04/ALD403-IHC-Poster-Baker-Smith-29-April-2015-for-Jim.pdf. Accessed 11 July 2019.
Dodick D, Goadsby PJ, Silberstein SD, et al. Randomized, double-blind, placebo-controlled trial of ALD403, an anti-CGRP antibody in the prevention of chronic migraine [poster]. In: European Headache and Migraine Trust International Congress; 2016.
Saper J, Lipton R, Kudrow D, et al. Primary results of PROMISE-1 (Prevention Of Migraine via Intravenous eptinezumab Safety and Efficacy–1) trial: a phase 3, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of eptinezumab for prevention of frequent episodic migraines [abstract no. S20.001]. Neurology. 2018;90(15 Suppl).
Kudrow D, Lipton R, Silberstein S, et al. Eptinezumab for prevention of chronic migraine: results of 2 infusions in the phase 3 PROMISE-2 (Prevention of Migraine via Intravenous Eptinezumab Safety and Efficacy–2) trial [abstract no. P2.10-006]. Neurology. 2019;92(15 Suppl).
Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425–34.
Raffaelli B, Mussetto V, Israel H, et al. Erenumab and galcanezumab in chronic migraine prevention: effects after treatment termination. J Headache Pain. 2019;20(1):66.
Goadsby PJ, Reuter U, Bonner J, et al. Phase 3, randomised, double-blind, placebo-controlled study to evaluate the efficacy and safety of erenumab (AMG 334) in migraine prevention: primary results of the STRIVE trial [abstract no. 62]. J Neurol Neurosurg Psychiatry. 2017;88(Suppl 1).
Dodick D, Ashina M, Kudrow D, et al. A phase 3, randomised, double-blind, placebo-controlled study to evaluate the efficacy and safety of erenumab in migraine prevention: primary results of the ARISE trial [abstract no. 63]. J Neurol Neurosurg Psychiatry. 2017;88(Suppl 1).
Novel drug approvals for 2018 (erenumab). https://www.fda.gov/drugs/developmentapprovalprocess/druginnovation/ucm592464.htm. Accessed 11 July 2019.
Highlights of prescribing information (erenumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf. Accessed 11 July 2019.
Farkkila M, Diener HC, Geraud G, et al. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11(5):405–13.
Ferrari MD, Farkkila M, Reuter U, et al. Acute treatment of migraine with the selective 5-HT1F receptor agonist lasmiditan: a randomised proof-of-concept trial. Cephalalgia. 2010;30(10):1170–8.
Kuca B, Siberstein SD, Wietecha L, et al. Lasmiditan is an effective acute treatment for migraine: a phase 3 randomized study. Neurology. 2018;91(24):e2222–32.
Wietecha LA, Kuca B, Adjei JA, et al. Phase 3 studies (SAMURAI, SPARTAN) of lasmiditan compared to placebo for acute treatment of migraine [abstract no. S50.008]. Neurology. 2018;90(15 Suppl).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Funding
No funding was received for the preparation of this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, A., Thangaraju, P. & Dhaneria, S. Recent and updated pharmacotherapy of migraine. Drugs Ther Perspect 35, 571–578 (2019). https://doi.org/10.1007/s40267-019-00664-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-019-00664-2