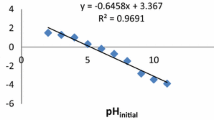
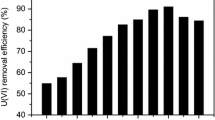
Abstract
The goal of this study was to use natural phosphate (NP) abundant in Algeria, as an adsorbent for the removal of uranium (VI) from aqueous solutions in batch adsorption. A full 23 factorial extended experimental design was investigated. The factors and levels used during the experiments were; pH (X1) (1–5), initial U(VI) concentration (X2) (30–60 mg L−1) and adsorbent dose (X3) (5–30 g L−1). The properties of NP were characterized by XRF, SEM, EDS, XRD and FTIR before and after adsorption. The effects of factors were explored by response surface methodology. The equilibrium data of U(VI) adsorption onto NP fitted to the Langmuir − 1 model at a maximum monolayer capacity of 11.11 mg g−1 with the kinetics being pseudo-second-order. The characterization of the filtered solid after adsorption revealed the formation of a new lamellar crystal phase of autunite Ca(UO2)2(PO4)2(H2O)6. The calculated value of the mean free energy indicates the chemisorption process. Under optimal conditions, the uranium effluent derived from the precipitation of ammonium uranyl carbonate removal performance of 100% was achieved.









Similar content being viewed by others
References
Anirudhan, T.; Lekshmi, G.; Shainy, F.: Synthesis and characterization of amidoxime modified chitosan/bentonite composite for the adsorptive removal and recovery of uranium from seawater. J. Colloid Interface Sci. 534, 248–261 (2019). https://doi.org/10.1016/j.jcis.2018.09.009
Jokinen, S.A.; Koho, K.; Virtasalo, J.; Jilbert, T.: Depth and intensity of the sulfate-methane transition zone control sedimentary molybdenum and uranium sequestration in a eutrophic low-salinity setting. Appl. Geochem. 122, 104767 (2020). https://doi.org/10.1016/j.apgeochem.2020.104767
Chung, Y.; Yun, Y.-M.; Kim, Y.-J.; Hwang, Y.; Kang, S.: Preparation of alumina-zirconia (Al-Zr) ceramic nanofiltration (NF) membrane for the removal of uranium in aquatic system. Water Supply 19, 789–795 (2019). https://doi.org/10.2166/ws.2018.123
Bjørklund, G.; Semenova, Y.; Pivina, L.; Dadar, M.; Rahman, M.M.; Aaseth, J.; Chirumbolo, S.: Uranium in drinking water: a public health threat. Arch. Toxicol. 94, 1551–1560 (2020). https://doi.org/10.1007/s00204-020-02676-8
Kornilov, A.; Piterkina, E.; Shcherbakova, K.; Makarov, A.; Dmitrieva, O.: Specific features of peroxide precipitation of uranium from acid water–ethanol solutions. Radiochemistry. 62, 173–176 (2020). https://doi.org/10.1134/S1066362220020046
Foster, R.I.; Amphlett, J.T.; Kim, K.-W.; Kerry, T.; Lee, K.; Sharrad, C.A.: SOHIO process legacy waste treatment: uranium recovery using ion exchange. J. Ind. Eng. Chem. 81, 144–152 (2020). https://doi.org/10.1016/j.jiec.2019.09.001
Hoyer, M.; Zabelt, D.; Steudtner, R.; Brendler, V.; Haseneder, R.; Repke, J.-U.: Influence of speciation during membrane treatment of uranium contaminated water. Sep. Purif. Technol. 132, 413–421 (2014). https://doi.org/10.1016/j.seppur.2014.05.044
Yang, X.; Zhang, Z.; Kuang, S.; Wei, H.; Li, Y.; Wu, G.; Geng, A.; Li, Y.; Liao, W.: Removal of thorium and uranium from leach solutions of ion-adsorption rare earth ores by solvent extraction with Cextrant 230. Hydrometallurgy 194, 105343 (2020). https://doi.org/10.1016/j.hydromet.2020.105343
Vakili, M.; Rafatullah, M.; Ibrahim, M.H.; Abdullah, A.Z.; Salamatinia, B.; Gholami, Z.: Oil palm biomass as an adsorbent for heavy metals. Rev. Environ. Contam. Toxicol. 232, 61–88 (2014). https://doi.org/10.1007/978-3-319-06746-9_3
Danish, M.; Hashim, R.; Ibrahim, M.M.; Rafatullah, M.; Sulaiman, O.: Surface characterization and comparative adsorption properties of Cr (VI) on pyrolysed adsorbents of Acacia mangium wood and Phoenix dactylifera L. stone carbon. J. Anal. Appl. Pyrol. 97, 19–28 (2012). https://doi.org/10.1016/j.jaap.2012.06.001
Oyekanmi, A.A.; Ahmad, A.; Hossain, K.; Rafatullah, M.: Adsorption of Rhodamine B dye from aqueous solution onto acid treated banana peel: Response surface methodology, kinetics and isotherm studies. PLoS ONE 14, e0216878 (2019). https://doi.org/10.1371/journal.pone.0216878
Oyekanmi, A.A.; Ahmad, A.; Hossain, K.; Rafatullah, M.: Statistical optimization for adsorption of Rhodamine B dye from aqueous solutions. J. Mol. Liq. 281, 48–58 (2019). https://doi.org/10.1016/j.molliq.2019.02.057
Khan, M.A.; Alqadami, A.A.; Otero, M.; Siddiqui, M.R.; Alothman, Z.A.; Alsohaimi, I.; Rafatullah, M.; Hamedelniel, A.E.: Heteroatom-doped magnetic hydrochar to remove post-transition and transition metals from water: synthesis, characterization, and adsorption studies. Chemosphere 218, 1089–1099 (2019). https://doi.org/10.1016/j.chemosphere.2018.11.210
Nibou, D.; Amokrane, S.: Catalytic performances of exchanged Y faujasites by Ce 3+, La 3+, UO 2 2+, Co 2+, Sr 2+, Pb 2+, Tl+ and NH 4+ cations in toluene dismutation reaction. Comput. Rend. Chim. 13, 527–537 (2010). https://doi.org/10.1016/j.crci.2009.12.006
Alahabadi, A.; Singh, P.; Raizada, P.; Anastopooulos, I.; Sivamani, S.; Dotto, G.L.; Landarani, M.; Ivanets, A.; Kyzas, G.Z.; Hosseini-Bandegharaei, A.: Activated carbon from wood wastes for the removal of uranium and thorium ions through modification with mineral acid Colloids Surf. Physicochem. Eng. Aspects 607, 125516 (2020). https://doi.org/10.1016/j.colsurfa.2020.125516
Cheira, M.F.; Kouraim, M.N.; Zidan, I.H.; Mohamed, W.S.; Hassanein, T.F.: Adsorption of U (VI) from sulfate solution using montmorillonite/polyamide and nano-titanium oxide/polyamide nanocomposites. J. Environ. Chem. Eng. 8, 104427 (2020). https://doi.org/10.1016/j.jece.2020.104427
Chen, B.; Wang, J.; Kong, L.; Mai, X.; Zheng, N.; Zhong, Q.; Liang, J.; Chen, D.: Adsorption of uranium from uranium mine contaminated water using phosphate rock apatite (PRA): isotherm, kinetic and characterization studies. Colloids Surf. Physicochem. Eng. Aspects 520, 612–621 (2017). https://doi.org/10.1016/j.colsurfa.2017.01.055
Li, Q.; Zhong, H.; Cao, Y.: Effects of the joint application of phosphate rock, ferric nitrate and plant ash on the immobility of As, Pb and Cd in soils. J. Environ. Manag. 265, 110576 (2020). https://doi.org/10.1016/j.jenvman.2020.110576
Yaacoubi, H.; Zidani, O.; Mouflih, M.; Gourai, M.; Sebti, S.: Removal of cadmium from water using natural phosphate as adsorbent. Procedia Eng. 83, 386–393 (2014). https://doi.org/10.1016/j.proeng.2014.09.039
Barka, N.; Assabbane, A.; Nounah, A.; Laanab, L.; Ichou, Y.A.: Removal of textile dyes from aqueous solutions by natural phosphate as a new adsorbent. Desalination 235, 264–275 (2009). https://doi.org/10.1016/j.desal.2008.01.015
Zhou, C.; Wang, X.; Song, X.; Wang, Y.; Fang, D.; Ge, S.; Zhang, R.: Insights into dynamic adsorption of lead by nano-hydroxyapatite prepared with two-stage ultrasound. Chemosphere 253, 126661 (2020). https://doi.org/10.1016/j.chemosphere.2020.126661
Guo, Y.; Gong, Z.; Li, C.; Gao, B.; Li, P.; Wang, X.; Zhang, B.; Li, X.: Efficient removal of uranium (VI) by 3D hierarchical Mg/Fe-LDH supported nanoscale hydroxyapatite: a synthetic experimental and mechanism studies. Chem. Eng. J. 392, 123682 (2020). https://doi.org/10.1016/j.cej.2019.123682
Skwarek, E.; Gładysz-Płaska, A.; Choromańska, J.; Broda, E.: Adsorption of uranium ions on nano-hydroxyapatite and modified by Ca and Ag ions. Adsorption 25, 639–647 (2019). https://doi.org/10.1007/s10450-019-00063-z
Simon, F.G.; Biermann, V.; Peplinski, B.: Uranium removal from groundwater using hydroxyapatite. Appl Geochem. 23, 2137–2145 (2008). https://doi.org/10.1016/j.apgeochem.2008.04.025
Rigali, M.J.; Brady, P.V.; Moore, R.C.: Radionuclide removal by apatite. Am. Miner. 101, 2611–2619 (2016). https://doi.org/10.2138/am-2016-5769
Diwan, V.; Sar, S.K.; Biswas, S.; Lalwani, R.: Adsorptive extraction of uranium (VI) from aqueous phase by dolomite. Groundw. Sustain. Dev. 11, 100424 (2020). https://doi.org/10.1016/j.gsd.2020.100424
Yang, H.; Luo, X.; Ding, H.; Zhang, X.: Adsorption of U (VI) by Elodea nuttallii: equilibrium, kinetic and mechanism analysis. J. Radioanal. Nucl. Chem. 319, 227–235 (2019). https://doi.org/10.1007/s10967-018-6346-7
Elnona, M.; Morci, E.; A. : Sorption of uranium on some natural modified clay mineral deposits. J. Agric. Sci. 27, 2329–2340 (2019). https://doi.org/10.21608/ajs.2019.16794.1084
Hu, W.; Zhang, Z.; Li, M.; Liu, H.; Zhang, C.; Chen, T.; Zhou, Y.: Enhanced uptake capacity for uranium (VI) in aqueous solutions by activated natural siderite: performance and mechanism. Appl. Geochem. 100, 96–103 (2019). https://doi.org/10.1016/j.apgeochem.2018.11.010
Sun, Z.; Chen, D.; Chen, B.; Kong, L.; Su, M.: Enhanced uranium (VI) adsorption by chitosan modified phosphate rock colloids surf. Physicochem. Eng. Aspects 547, 141–147 (2018). https://doi.org/10.1016/j.colsurfa.2018.02.043
Acknowledgements
My deep and sincere thanks to all authors who contributed directly or indirectly to the realization of this work. Special thanks to all staff of the Nuclear Research Center Draria, Materials Technology Laboratory of Bab Ezzouar and the Department of Civil, Environmental, Land, Building Engineering and Chemistry of Technical University of Bari Italy for their help and benevolence each in his area of expertise.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ouassel, S., Chegrouche, S., Nibou, D. et al. Adsorption of Uranium (VI) onto Natural Algerian Phosphate: Study of Influencing Factors, and Mechanism. Arab J Sci Eng 46, 6645–6661 (2021). https://doi.org/10.1007/s13369-020-05299-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-05299-4