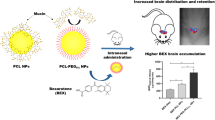
Abstract
There is an opinion in the medical associations that intranasal administration of medicine allows direct olfactory transfer of drugs into the central nervous system bypassing the blood–brain barrier. This approach could be a valuable solution to the problem of cerebral pathology treatment. We propose a new system of microcontainers for the delivery of an active component to the brain by intranasal administration. The microcontainers were fabricated on the base of porous calcium carbonate particles modified with mucoadhesive biocompatible polymer or polymer/surfactant coating. Loperamide was encapsulated in the proposed microcontainers as a model drug, which cannot pass the blood–brain barrier. The efficiency of microcontainers loaded with the anesthetic loperamide has been assessed by the formalin test in rats in vivo. The results of the in vivo experiments demonstrate decrease in the pain sensitivity after intranasal administration of proposed system, and benefit of mucoadhesive biocompatible coating aiming to improve the anesthetic effect.







Similar content being viewed by others
References
Pathak YV, editor. (2009). Handbook of nutraceuticals volume I: ingredients, formulations, and applications. Handbook of nutraceuticals volume I: ingredients, formulations, and applications. p. 292–306.
Graham, N., Steiner, T.J., & Kesserling, J. (2007). Neurological disorders: Public health challenges (p. 28–37). Geneva, Switzerland: WHO, WHO Press.
Pond, S. M., & Tozer, T. N. (1984). First-pass elimination. Basic concepts and clinical consequences. Clinical Pharmacokinetics, 9(1), 1–25.
Sakane, T., Akizuki, M., Yoshida, M., Yamashita, S., Nadai, T., Hashida, M., et al. (1991). Transport of cephalexin to the cerebrospinal fluid directly from the nasal cavity. Journal of Pharmacy and Pharmacology, 43(6), 449–51.
Banks, W. A., During, M. J., Niehoff, M. L. (2004). Brain uptake of the glucagon-like peptide-1 antagonist exendin(9-39) after intranasal administration. Journal of Pharmacology and Experimental Therapeutics, 309(2), 469–75.
Westin, U. E., Boström, E., Gråsjö, J., Hammarlund-Udenaes, M., Björk, E. (2006). Direct nose-to-brain transfer of morphine after nasal administration to rats. Pharmaceutical Research, 23(3), 565–72.
Jadhav, K., Gambhire, M., Shaikh, I., Kadam, V., Pisal, S. (2007). Nasal drug delivery system-factors affecting and applications. Current Drug Theraphy, 2(1), 27–38.
Mathison, S., Nagilla, R., Kompella, U. B. (1998). Nasal route for direct delivery of solutes to the central nervous system: fact or fiction? Journal of Drug Targeting, 5(6), 415–41.
Illum, L. (2004). Is nose-to-brain transport of drugs in man a reality? Journal of Pharmacy and Pharmacology, 56(1), 3–17.
Read, R. C., Naylor, S. C., Potter, C. W., Bond, J., Jabbal-Gill, I., Fisher, A., et al. (2005). Effective nasal influenza vaccine delivery using chitosan. Vaccine, 23(35), 4367–74.
Hinchcliffe, M., & Illum, L. (1999). Intranasal insulin delivery and therapy. Advanced Drug Delivery Reviews, 35(2–3), 199–234.
Anand Kumar, T. C., David, G. F., Umberkoman, B., Saini, K. D. (1974). Uptake of radioradioactivity by body fluids and tissues in rhesus monkeys after intravenous injection or intranasal spray of tritium-labelled estradiol and progesterone. Current Science, 43(14), 435–9.
Trushina, D. B., Bukreeva, T. V., Kovalchuk, M. V., Antipina, M. N. (2014). CaCO3 vaterite microparticles for biomedical and personal care applications. Materials Science and Engineering, 45, 644–58.
Donath, E., Sukhorukov, G. B., Caruso, F., Davis, S. A., Möhwald, H. (1998). Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes. Angew Chemie International Edition, 37(16), 2201–5.
Yi, Q., & Sukhorukov, G. B. (2013). Externally triggered dual function of complex microcapsules. ACS Nano, 7(10), 8693–705.
Gai, M., Frueh, J., Girard-Egrot, A., Rebaud, S., Doumeche, B., He, Q. (2015). Micro-contact printing of PEM thin films: effect of line tension and surface energies. RSC Advances, 5(64), 51891–9.
Volodkin, D. (2014). CaCO3 templated micro-beads and -capsules for bioapplications. Advance in Colloid and Interface Science, 207, 306–24.
Gorin, D. A., Portnov, S. A., Inozemtseva, O. A., Luklinska, Z., Yashchenok, A. M., Pavlov, A. M., et al. (2008). Magnetic/gold nanoparticle functionalized biocompatible microcapsules with sensitivity to laser irradiation. Physical Chemistry Chemical Physics, 10(45), 6899–905.
Andreeva, D. V., Gorin, D. A., Shchukin, D. G., Sukhorukov, G. B. (2006). Magnetic microcapsules with low permeable polypyrrole skin layer. Macromolecular Rapid Communications, 27(12), 931–6.
Gai, M., Frueh, J., Hu, N., Si, T., Sukhorukov, G. B., He, Q. (2016). Self-propelled two dimensional polymer multilayer plate micromotors. Physical Chemistry Chemical Physics, 18(5), 3397–401.
Walker, D., Kasdorf, B. T., Jeong, H.-H., Lieleg, O., Fischer, P. (2015). Enzymatically active biomimetic micropropellers for the penetration of mucin gels. Science Advances, 1(11), e1500501–e1500501.
He, W., Frueh, J., Wu, Z., He, Q. (2016). How leucocyte cell membrane modified janus microcapsules are phagocytosed by cancer cells. ACS Applied Materials & Interfaces, 8(7), 4407–15.
Yan, L., Ehrlich, P. J., Gibson, R., Pickett, C., Beckman, R. A. (2009). How can we improve antibody-based cancer therapy? MAbs, 1(1), 67–70.
Heyder, J., Gebhart, J., Rudolf, G., Schiller, C. F., Stahlhofen, W. (1986). Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. Journal of Aerosol Science, 17(5), 811–25.
Shang, Y. D., Inthavong, K., Tu, J. Y. (2015). Detailed micro-particle deposition patterns in the human nasal cavity influenced by the breathing zone. Computers & Fluids, 114, 141–50.
Hatch, T. F. (1961). Distribution and deposition of the inhaled particles in respiratory tract. Bacteriological Reviews, 25(3), 237–40.
Stuart, B. O. (1973). Deposition of inhaled aerosols. Archives of Internal Medicine, 31(1), 60–73.
Volodkin, D. V., Petrov, A. I., Prevot, M., Sukhorukov, G. B. (2004). Matrix polyelectrolyte microcapsules: new system for macromolecule encapsulation. Langmuir, 20(8), 3398–406.
Soane, R., Frier, M., Perkins, A., Jones, N., Davis, S., Illum, L. (1999). Evaluation of the clearance characteristics of bioadhesive systems in humans. International Journal of Pharmaceutics, 178(1), 55–65.
Kreuter, J., Shamenkov, D., Petrov, V., Ramge, P., Cychutek, K., Koch-Brandt, C., et al. (2002). Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood–brain barrier. Journal of Drug Targeting, 10(4), 317–25.
Roldungin VI (2008). Physical chemistry of the surfaces: a textbook—monography. Dolgoprudny: Publishing House «Intellect»; p. 568.
Ariga, K., Lvov, Y. M., Kawakami, K., Ji, Q., Hill, J. P. (2011). Layer-by-layer self-assembled shells for drug delivery. Advanced Drug Delivery Reviews, 63(9), 762–71.
Washington, N., Steele, R. J., Jackson, S., Bush, D., Mason, J., Gill, D., et al. (2000). Determination of baseline human nasal pH and the effect of intranasally administered buffers. International Journal of Pharmaceutics, 198(2), 139–46.
Jones, N. (2001). The nose and paranasal sinuses physiology and anatomy. Advanced Drug Delivery Reviews, 51(1–3), 5–19.
Shutava, T. G., Pattekari, P. P., Arapov, K. A., Torchilin, V. P., Lvov, Y. M. (2012). Architectural layer-by-layer assembly of drug nanocapsules with PEGylated polyelectrolytes. Soft Matter, 8(36), 9418.
Matthies, B. K., & Franklin, K. B. J. (1995). Effects of partial decortication on opioid analgesia in the formalin test. Behavioural Brain Research, 67(1), 59–66.
Rosland, J. H., Tjølsen, A., Mæhle, B., Hole, K. (1990). The formalin test in mice: effect of formalin concentration. Pain, 42(2), 235–42.
Borodina, T., Markvicheva, E., Kunizhev, S., Möhwald, H., Sukhorukov, G. B., Kreft, O. (2007). Controlled release of DNA from self-degrading microcapsules. Macromolecular Rapid Communications, 28(18–19), 1894–9.
Matthies, B. K., & Franklin, K. B. J. (1992). Formalin pain is expressed in decerebrate rats but not attenuated by morphine. Pain, 51(2), 199–206.
Acknowledgments
We gratefully acknowledge the group of Prof. N.V. Gulyaeva (Functional Biochemistry of the nervous system Lab) for the performing of the in vivo experiments in the Institute of Higher Nervous Activity and Neurophysiology, Russian Academy of Sciences. This study was performed using the equipment of the Shared Research Center of the Institute of Crystallography of the Russian Academy of Sciences and partially funded by the Russian Foundation for Basic Research and Moscow city Government according to the research project no. 15-33-70032 «mol_a_mos».
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borodina, T.N., Trushina, D.B., Marchenko, I.V. et al. Calcium Carbonate-Based Mucoadhesive Microcontainers for Intranasal Delivery of Drugs Bypassing the Blood–Brain Barrier. BioNanoSci. 6, 261–268 (2016). https://doi.org/10.1007/s12668-016-0212-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-016-0212-2