Abstract
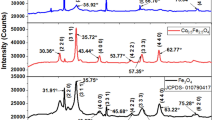
Fe3O4 nanoparticles were synthesized, using a simple co-precipitation method and then calcined at various temperatures in the range of 50–850 °C for 1 h in air. After calcination, the nanoparticles were characterized by X-ray diffraction, scanning electron microscopy, Fourier transform infrared spectroscopy and vibrating sample magnetometer. The X-ray diffraction results indicated that Fe3O4 nanoparticles were converted to γ-Fe2O3 by calcining at 250 °C for 1 h and then to α-Fe2O3 on calcining in the range of 550–650 °C. The average crystallite size of the nanoparticles was calculated by using the Scherrer and Williamson-Hall methods. The average crystallite size of the iron oxides NPs increased from 7.2 to 35.8 nm by increasing calcination temperature from 50 to 850 °C. A small strain existed, which were affected on the physical and structural properties of Fe3O4. The vibrating sample magnetometer results indicated that, the as-synthesized nanoparticles converted from superparamagnetic to ferromagnetic phase with calcinations up to 650 °C, due to increasing size of nanoparticles from a single domain to multidomain as indicated in the X-ray diffraction results.
Graphical Abstract







Similar content being viewed by others
References
M Aliahmad and M Noori Indian J. Phys. 87 431 (2013).
G Mandal and T Ganguly Indian J. Phys. 85 1229 (2011).
S Mitra et al. Indian J. Phys. 85 649 (2011).
S Devi and M Srivastva Indian J. Phys. 84 1561 (2010).
J Bhadra and D Sarkar Indian J. Phys. 84 693 (2010).
S Sarmah and A Kumar Indian J. Phys. 85 713 (2011).
M Hofmann, S J Campbell, W A Kaczmarek and S Welzel J. Alloys Comp. 348 278 (2003).
R Valenzuela et al. J. Alloys Comp. 488 227 (2009).
W H Bragg Nature 95 561 (1915).
H T Jeng, G Y Guo and D J Huang Phys. Rev. Lett. 93 156403 (2004).
E J Verwey, P W Haayman and F C Romeijn J. Chem. Phys. 15 181 (1947).
S Laurent et al. Chem. Rev. 108 2064 (2008).
M Chirita and I Grozescu Chem. Bull. POLITECHNICA Univ. 54 1 (2009).
J Tang, M Myers, K A Bosnick and L E Brus J. Phys. Chem. B 107 7501 (2003).
Y S Kim, K Nakatsuka, T Fujita and T Atarashi J. Magn. Magn. Mater. 201 361 (1999).
J H Li, R Y Hong, H Z Li, J Ding, Y Zheng and D G Wei Mater. Chem. Phys. 113 140 (2009).
J H Jang and H B Lim Microchem. J. 94 148 (2010).
P Mallick, C Rath, R Biswal and N C Mishra Indian J. Phys. 83 517 (2009).
C R Lin, Y M Chu and S C Wang Mater. Lett. 60 447 (2006).
S Ayyappan, G Gnanaprakash, G Panneerselvam, M P Antony and J Philip J. Phys. Chem. C 112 18376 (2008).
S Duhan and S Devi Int. J. Electron. Eng. 2 89 (2010).
G Gnanaprakash, S Ayyappan, T Jayakumar, J Philip and B Raj Nanotech. 17 5851 (2006).
A Jafari, K Boustani and S Farjami Shayesteh J. Supercond. Nov. Magn. 27 187 (2014).
A A Shal and A Jafari J. Supercond. Nov. Magn. 27 1531 (2014).
H E Swanson, H F McMurdie, M C Morris and E H Evans Standard X-Ray Diffraction Powder Patterns (Washington: National Bureau of Standards) Vol 25, Sec 5, p 31 (1967).
H Iida, K Takayanagi, T Nakanishi and T Osaka J. Colloid. Interface. Sci. 314 274 (2007).
R M Cornell and U Schwertmann The iron oxides. Structure, Properties, Reactions, Occurrences and Uses (Weinheim: John Wiley & Sons) 2nd ed. p 5 (2006).
A Maurya, P Chauhan, SK Mishra and R K Srivastava J. Alloys Comp. 509 8433 (2011).
C Kittel Introduction to Solid State Physics (Singapore: John Wiley & Sons) 7th ed. p 42 (1996).
B D Cullity Elements of X-ray Diffraction (Massachusetts Addison-Wesley) 1st ed. p 465 (1956).
A K Zak, W H Abd Majid, M E Abrishami and R Yousefi Solid State Sci. 13 251 (2011).
M Nirouei, A Jafari and K Boustani J. Supercond. Nov. Magn. 27 1 (2014).
A K Zak and W H Abd Majid Ceram. Int. 36 1905 (2010).
M Riazian Indian J. Phys. 87 991 (2013).
V Senthilkumar, P Vickraman, M Jayachandran and C Sanjeeviraja J. Mater. Sci.: Mater. Electron. 21 343 (2010).
C Bharti, S N Choudhary and T P Sinha Indian J. Phys. 83 409 (2009).
T P Rao, M C Santhosh Kumar and V Ganesan Indian J. Phys. 85 1381 (2011).
A K Tripathi, M K Singh, M C Mathpal, S K Mishra and A Agarwal J. Alloys Comp. 549 114 (2013).
S Nasrazadani and A Raman Corros Sci. 34 1355 (1993).
M Ishii and M Nakahira Solid State Commun. 11 209 (1972).
M Ma, Y Zhang, W Yu, H-y Shen, H-q Zhang and N Gu Colloid. Surfaces A: Physicochem. Eng. Aspects 212 219 (2003).
J L Zhang, R S Srivastava and R D K Misra Langmuir 23 6342 (2007).
H Namduri and S Nasrazadani Corros. Sci. 50 2493 (2008).
F Dang, N Enomoto, J Hojo and K Enpuku Ultrason. Sonochem. 16 649 (2009).
A Raman, B Kuban and A Razvan Corros. Sci. 32 1295 (1991).
D M Farrell A Study of The Infrared Absorption in The Oxidation of Magnetite to Maghemite and Hematite (Canada Mines Branch) Inv. Rept. 72–18 p 44 (1972).
Y S Li, J S Church and A L Woodhead J. Magn. Magn. Mater. 324 1543 (2012).
R Kaiser and G Miskolczy J. Appl. Phys. 41 1064 (1970).
S Ahmad, U Riaz, A Kaushik and J Alam J. Inorg. Organomet. Polym. 19 355 (2009).
S P Gubin Magnetic Nanoparticles (Weinheim: Wiley-VCH) p 214 (2009).
R D Zysler, M Vasquez-Mansilla, C Arciprete, M Dimitrijewits, D Rodriguez-Sierra and C Saragovi J. Magn. Magn. Mater. 224 39 (2001).
Acknowledgments
We gratefully acknowledge university of Guilan for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jafari, A., Farjami Shayesteh, S., Salouti, M. et al. Dependence of structural phase transition and lattice strain of Fe3O4 nanoparticles on calcination temperature. Indian J Phys 89, 551–560 (2015). https://doi.org/10.1007/s12648-014-0627-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-014-0627-y