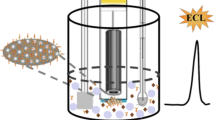
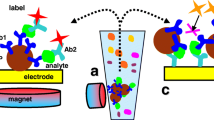
Abstract
Electrochemical methods are increasingly applied to immunoassays, because they overcome problems associated with other modes of detection. In particular, with respect to conventional immunoassays, electrochemical immunosensors show versatility, reliability, and fast analysis time. In immunosensor strategy, the antigen or antibody can be immobilized directly onto the surface of the electrochemical transducer that will finally be used to reveal the amount of the affinity reaction. However, the use of the electrode surface as a solid phase as well as an electrochemical transducer presents some problems: a shielding of the surface by biospecifically bound antibody molecules can cause hindrance in the electron transfer, resulting in a reduced voltammetric signal. Thus, as an alternative solid phase, magnetic beads because of their low toxicity and high biocompatibility have gained much attention in chemistry, associated with various analytical techniques, due to their suitability for immobilization of biomolecules. Magnetic micro- or nanobeads can be separated easily and quickly by magnetic forces and will be used together with bioaffine ligands, e.g., antibodies or proteins with a high affinity to the target. The special advantages of magnetic separation techniques are the fast and simple handling of a sample vial and the opportunity to deal with large sample volumes without the need for time-consuming centrifugation steps. This also makes biomagnetic separation ideal for automated assay/analysis systems which will play a very important role in the near future. This review presents some examples of immunochemical assay developed using magnetic beads as a solid phase coupled with electrochemical detection techniques, in particular, using electrochemical arrays as transducers. Applications related to static measurements, together with in-flow detection systems are presented.













Similar content being viewed by others
References
Franek M, Hruska K (2005) Antibody based methods for environmental and food analysis: a review. Vet Med Czech 50:1–10
Li X-M, Yang X-Y, Zhang S-S (2008) Electrochemical enzyme immunoassay using model labels. Trends Anal Chem 27:543–553
Liu JM, Zhu GH, Rao ZM, Wei CJ, Li LD, Chen CL, Li ZM (2005) Determination of human IgG by solid substrate room temperature phosphorescence immunoassay based on an antibody labeled with nanoparticles containing dibromofluorescein luminescent molecules. Anal Chim Acta 528:29–35
Beckman EM, Kawaguchi T, Chandler GT, Decho AW (2008) Development of a microplate-based fluorescence immunoassay using quantum dot streptavidin conjugates for enumeration of putative marine bacteria, Alteromonas sp., associated with a benthic harpacticoid copepod. J Microbiol Meth 75:441–444
Takahashi H, Takahashi M, Nagaya H, Hirako M, Sawai K, Minamihashi A, Inumaru S, Yokomizo Y, Geshi M, Okano A, Okuda K (2005) Establishment of a specific radioimmunoassay for bovine interferon τ. Theriogenology 63(4):1050–1060
Fowler JM, Wong DKY, Halsall HB, Heineman WR (2008) Recent developments in electrochemical immunoassays and immunosensors. In: Zhang X, Ju H, Wang J (eds) Electrochemical Sensors. Biosensors and their Biomedical Applications. Elsevier, New York, pp 115–143
Laschi S, Mascini M (2002) Disposable electrochemical immunosensor for environmental applications. Ann Chim 92:425–433
Ding Y, Zhou L, Halsall HB, Heineman WR (1999) Feasibility studies of simultaneous multianalyte amperometric immunoassay based on spatial resolution. J Pharm Biomed Anal 19(1–2):153–161
Zaytseva N, Montagna RA, Baeumner AJ (2005) Microfluidic Biosensor for the Serotype-Specific Detection of Dengue Virus RNA. Anal Chem 77:7520–7527
Kwon Y, Hara CA, Knize MG, Hwang MH, Venkateswaran KS, Wheeler EK, Bell PM, Renzi RF, Fruetel JA, Bailey CG (2008) Magnetic bead based immunoassay for autonomous detection of toxins. Anal Chem 80:8416–8423
Thomas JH, Kim SK, Hesketh PJ, Halsall HB, Heineman WR (2004) Microbead-based electrochemical immunoassay with interdigitated array electrodes. Anal Biochem 328:113–122
Bausch AR, Möller W, Sackmann E (1999) Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. Biophys J 76:573–579
Kuramitz H (2009) Magnetic microbead-based electrochemical immunoassays. Anal Bioanal Chem 394:61–69
Sugawara K, Yugami A, Kuramitz H (2009) Electrochemical monitoring of binding between wheat germ agglutinin and cellohexose-modified magnetic microbeads. Anal Bioanal Chem 395:767–772
Zacco E, Pividori MI, Alegret S, Galve R, Marco M-P (2006) Electrochemical magnetoimmunosensing strategy for the detection of pesticides residues. Anal Chem 78:1780–1788
Centi S, Laschi S, Fránek M, Mascini M (2005) A disposable immunomagnetic electrochemical sensor based on functionalised magnetic beads and carbon-based screen-printed electrodes (SPCEs) for the detection of polychlorinated biphenyls (PCBs). Anal Chim Acta 538:205–212
Centi S, Silva E, Laschi S, Palchetti I, Mascini M (2007) Polychlorinated biphenyls (PCBs) detection in milk samples by an electrochemical magneto-immunosensor (EMI) coupled to solid-phase extraction (SPE) and disposable low-density arrays. Anal Chim Acta 594:9–16
Lin Y-Y, Liu G, Wai CM, Lin Y (2007) Magnetic beads-based bioelectrochemical immunoassay of polycyclic aromatic hydrocarbons. Electrochem Commun 9:1547–1552
Tudorache M, Tencaliec A, Bala C (2008) Magnetic beads-based immunoassay as a sensitive alternative for atrazine analysis. Talanta 77:839–843
Varlan R, Suls J, Jacobs P, Sansen W (1995) A new technique of enzyme entrapment for planar biosensors. Biosens Bioelectron 10(8):XV–XIX
Dock E, Christenson A, Sapelnikova S, Krejci J, Emnéus J, Ruzgas T (2005) A steady-state and flow-through cell for screen-printed eight-electrode arrays. Anal Chim Acta 531:165–172
Stefan R-I, Van Staden J, Aboul-Enein HY (1999) Electrochemical sensor arrays. Crit Rev Anal Chem 29:133–153
Tang D, Tang J, Su B, Ren J, Chen G (2010) Simultaneous determination of five-type hepatitis virus antigens in 5 min using an integrated automatic electrochemical immunosensor array. Biosens Bioelectron 25:1658–1662
Kim SJ, Gobi KV, Iwasaka H, Tanaka H, Miura N (2007) Novel miniature SPR immunosensor equipped with all-in-one multi-microchannel sensor chip for detecting low-molecular-weight analytes. Biosens Bioelectron 23:701–707
Morais S, Tortajada-Genaro LA, Arnandis-Chover T, Puchades R, Maquieira A (2009) Multiplexed microimmunoassays on a digital versatile disk. Anal Chem 81:5646–5654
Wu J, Yan F, Tang J, Zhai C, Ju H (2007) A disposable multianalyte electrochemical immunosensor array for automated simultaneous determination of tumor markers. Clin Chem 53:1495–1502
Tang D, Yuan R, Chai Y (2007) Magnetic control of an electrochemical microfluidic device with an arrayed immunosensor for simultaneous multiple immunoassay. Clin Chem 53(7):1323–1329
Thomas JH, Ronkainen-Matsuno NJ, Farrell S, Halsall HB, Heineman WR (2003) Microdrop analysis of a bead-based immunoassay. Microchem J 74:267–276
Kálab T, Skládal P (1997) Disposable multichannel immunosensors for 2, 4-dichlorophenoxyacetic acid using acetylcholinesterase as an enzyme label. Electroanalysis 9:293–297
Kálab T, Skládal P (1995) A multichannel immunochemical sensor for determination of 2, 4-dichlorophenoxyacetic acid. Anal Chim Acta 316:73–78
Dequaire M, Degrand C, Limoges B (1999) An immunomagnetic electrochemical sensor based on a perfluorosulfonate-coated screen-printed electrode for the determination of 2, 4-dichlorophenoxyacetic acid. Anal Chem 71:2571–2577
Peruski AH, Peruski LF Jr (2003) Immunological methods for detection and identification of infectious disease and biological warfare agents. Clin Diagn Lab Immunol 10:506–513
Zhuang J, Cheng T, Gao L, Luo Y, Ren Q, Lu D, Tang F, Ren X, Yang D, Feng J, Zhu J, Yan X (2010) Silica coating magnetic nanoparticle-based silver enhancement immunoassay for rapid electrical detection of ricin toxin. Toxicon 55:145–152
Gatto-Menking DL, Yu H, Bruno JG, Goode MT, Miller M, Zulich AW (1995) Sensitive detection of biotoxoids and bacterial spores using an immunomagnetic electrochemiluminescence sensor. Biosens Bioelectron 10:501–507
Centi S, Stoica AI, Laschi S, Mascini M (2010) Development of an electrochemical immunoassay based on the use of an eight-electrodes screen-printed array coupled with magnetic beads for the detection of antimicrobial sulfonamides in honey. Electroanalysis. doi:10.1002/elan.200900618
Varshney M, Li Y (2007) Interdigitated array microelectrode based impedance biosensor coupled with magnetic nanoparticle–antibody conjugates for detection of Escherichia coli O157:H7 in food samples. Biosens Bioelectron 22:2408–2414
Varshney M, Li Y, Srinivasan B, Tung S (2007) A label-free, microfluidics and interdigitated array microelectrode-based impedance biosensor in combination with nanoparticles immunoseparation for detection of Escherichia coli O157:H7 in food samples. Sensors Actuators B 128:99–107
Delibato E, Volpe G, Romanozzo D, De Medici D, Toti L, Moscone D, Palleschi G (2009) Development and application of an electrochemical plate coupled with immunomagnetic beads (ELIME) array for Salmonella enterica detection in meat samples. J Agric Food Chem 57:7200–7204
Piermarini S, Volpe G, Micheli L, Moscone D, Palleschi G (2009) An ELIME-array for detection of aflatoxin B1 in corn samples. Food Control 20:371–375
Pumera M, Merkoci A, Alegret S (2006) New materials for electrochemical sensing VII. Microfluidic chip platforms. Trends. Anal Chem 25:219–235
Rossier JS, Baranek S, Morier P, Vollet C, Vulliet F, De Chastonay Y, Reymond F (2008) GRAVI: robotized microfluidics for fast and automated immunoassays in low volume. JALA 13(6):322–329
Lin C-C, Wang J-H, Wu H-W, Lee G-B (2010) Microfluidic Immunoassays. JALA 15(3):253–274
Lien KY, Lee WC, Lei HY, Lee GB (2007) Integrated reverse transcription polymerase chain reaction systems for virus detection. Biosens Bioelectron 22(8):1739–1748
Qiua J, Zhoub Y, Chenb H, Lina JM (2009) Immunomagnetic separation and rapid detection of bacteria using bioluminescence and microfluidics. Talanta 79(3):787–795
Hoegger D, Morier P, Vollet C, Heini D, Reymond F, Rossier JS (2007) Disposable microfluidic ELISA for the rapid determination of folic acid content in food products. Anal Bioanal Chem 387:267–275
Berti F, Laschi S, Palchetti I, Rossier JS, Reymond F, Mascini M, Marrazza G (2009) Microfluidic-based electrochemical genosensor coupled to magnetic beads for hybridization detection. Talanta 77:971–978
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laschi, S., Centi, S. & Mascini, M. Electrochemical arrays coupled with magnetic separators for immunochemistry. Bioanal Rev 3, 11–25 (2011). https://doi.org/10.1007/s12566-010-0020-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12566-010-0020-z