Abstract
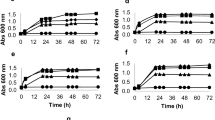
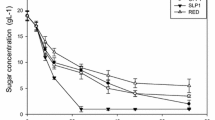
The hydrolysis which converts polysaccharides to the fermentable sugars for yeast’s lingocellulosic ethanol production also generates byproducts which inhibit the ethanol production. To investigate the extent to which inhibitory compounds affect yeast’s growth and ethanol production, fermentations by Saccharomyces cerevisiae K35 were investigated in various concentrations of acetic acid, furfural, 5-hydroxymethylfurfural (5-HMF), syringaldehyde, and coumaric acid. Fermentation in hydrolysates from yellow poplar and waste wood was also studied. After 24 h, S. cerevisiae K35 produced close to theoretically predicted ethanol yields in all the concentrations of acetic acid tested (1 ∼ 10 g/L). Both furans and phenolics inhibited cell growth and ethanol production. Ethanol yield, however, was unaffected, even at high concentrations, except in the cases of 5 g/L of syringaldehyde and coumaric acid. Although hydrolysates contain various toxic compounds, in their presence, S. Cerevisiae K35 consumed close to all the available glucose and yielded more ethanol than theoretically predicted. S. Cerevisiae K35 was demonstrated to have high tolerance to inhibitory compounds and not to need any detoxification for ethanol production from hydrolysates.
Similar content being viewed by others
References
Talebnia, F., C. Niklasson, and M. J. Taherzadeh (2005) Ethanol production from glucose and dilute-acid hydrolyzates by encapsulated S. cerevisiae. Biotechnol. Bioeng. 90: 345–353.
Martín, C. and L. J. Jönsson (2003) Comparison of the resistance of industrial and laboratory strains of Saccharomyces and Zygosaccharomyces to lignocelluloses-derived fermentation. Enz. Microb. Tech. 32: 386–395.
Palmqvist, E. and B. Hahn-Hägerdal (2000) Fermentation of lignocellulosic hydrolysates II: inhibitors and mechanisms of inhibition. Bioresource Technol. 74: 25–33.
Parajó, J. C., H. Domínguez, and J. M. Domínguez (1998) Biotechnological production of xylitol. Part 3: Operation in culture media made from lignocelluloses hydrolysates. Bioresource Technol. 66: 25–40.
Almeida, J. R., T. Modig, A. Petersson, B. Hähn-Hägerdal, G. Lidén, and M. F. Gorwa-Grauslund (2007) Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biot. 82: 340–349.
Sakai, S., Y. Tsuchida, S. Okino, O. Ichihashi, H. Kawaguchi, T. Watanabe, M. Inui, and H. Yukawa (2007) Effect of lignocellulose-derived Inhibitors on growth of and ethanol production by growth-arrested Corynebacterium glutamicum R. Appl. Environ. Microb. 73: 2349–2353.
Dunlop, A. P. (1948) Furfural formation and behavior. J. Ind. Eng. Chem. 40: 204–209.
Ulbricht, R. J., S. J. Northup, and J. A. Thomas (1984) A review of 5-hydroxymethylfurfural (HMF) in parenteral solutions. Fund. Appl. Toxicol. 4: 843–853.
Popoff, T. and O. Theander (1976) Formation of aromatic compounds from carbohydrates. Acta Chem. Scand. 30: 397–402.
Kötter, P. and M. Ciriacy (1993) Xylose fermentation by Saccharomyces cerevisiae. Appl. Microbiol. Biot. 38: 776–783.
Hahn-Hägerdal, B., C. F. Wahlbom, M., Gárdonyi, W. H. van Zyl, R. R. Cordero Otero, and L. J. Jönsson (2001) Metabolic engineering of Saccharomyces cerevisiae for xylose utilization. Adv. Biochem. Eng. Biot. 73: 53–84.
Lee, M., D. H. Cho, Y. H. Kim, J. Lee, J. H. Lee, S. W. Kim, J. Cho, D. Lee, S. Kim, and C. Park (2009) Effect of biomassderived inhibitors on ethanol production. Kor. Soc. Biotechnol. Bioeng. J. 24: 439–445.
Kim, T. -G. and K. Kim (1996) The construction of a stable starch-fermenting yeast strain using genetic engineering and raremating. Appl. Biochem. Biotech. 59: 39–51.
Singleton, V. L., R. Orthofer, and R. M. Lamuela-Raventos (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method. Enzymol. 299: 152–178.
Pampulha, M. E. and M. C. Loureiro-Dias (1989) Combined effect of acetic acid, pH, and ethanol on intracellular pH of fermenting yeast. Appl. Microbiol. Biot. 31: 547–550.
Verduyn, C., E. Postma, W. A. Scheffers and J. P. Van Dijken (1992) Effect of benzoic acid on metabolic fluxes in yeasts: A continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8: 501–517.
Russell, J. B. (1992) Another explanation for the toxicity of fermentation acids at low pH: Anion accumulation versus uncoupling. J. Appl. Bacteriol. 73: 363–370.
Taherzadeh, M. J., L. Gustafsson, C. Niklasson, and G. Lidén (2000) Inhibition effects of furfural on aerobic batch cultivation of Saccharomyces cerevisiae growing on ethanol and/or acetic acid. J. Biosci. Bioeng. 90: 374–380.
Modig, T., G. Lidén, and M. J. Taherzadeh (2002) Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase, and pyruvate dehydrogenase. Biochem. J. 363: 769–776.
Horváth, I. S., C. J. Franzén, M. J. Taherzadeh, C. Niklasson, and G. Lidén (2003) Effects of furfural on the respiratory metabolism of Saccharomyces cerevisiae in glucose-limited chemostats: Metabolic modeling of furfural conversion. Appl. Environ. Microb. 69: 4076–4086.
Gorsich, S. W., P. J. Slininger, and J. Mccaffery (2006) The fermentation inhibitor furfural causes cellular damage to Saccharomyces cerevisiae. Proceedings of 28 th Symposium on Biotechnology for Fuels and Chemicals. April 30–May 3. Nashville. USA.
Heipieper, H. J., F. J. Weber, J. Sikkema, H. Keweloh, and J. A. M. de Bont (1994) Mechanisms of resistance of whole cells to toxic organic solvents. Trends Biotechnol. 12: 409–415.
Terada, H. (1990) Uncouplers of oxidative phosphorylation. Environ. Health Persp. 87: 213–218.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, H., Cho, D.H., Kim, Y.H. et al. Tolerance of Saccharomyces cerevisiae K35 to lignocellulose-derived inhibitory compounds. Biotechnol Bioproc E 16, 755–760 (2011). https://doi.org/10.1007/s12257-010-0474-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-010-0474-4