Abstract
Introduction
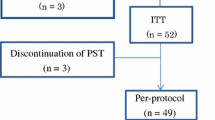
To evaluate the sequential administration of doxorubicin (A) and cyclophosphamide (C) followed by weekly docetaxel in women with stage II to IIIA breast cancer.
Patients and methods
Patients received 60 mg/m2 of A and 600 mg/m2 of C every three weeks for four cycles followed by 12 infusions of weekly docetaxel at a dose of 36 mg/m2 and with a 2-week resting period.
Results
Sixty-three women were included. On an intentionto-treat basis, clinical response rate was 90% (95% CI: 83–98), with 46% complete responses. Breast-conserving surgery could be performed in 43 patients (68%). Complete pathological responses in the breast were confirmed in 17% of patients. No correlations between levels of expression of topoisomerase II alpha, survivin or p27 and the pathological response were detected. The study treatment was generally well tolerated.
Conclusion
Neoadjuvant AC followed by weekly docetaxel is a feasible regimen for patients with early-stage breast cancer.
Similar content being viewed by others
References
Nabholtz JM, Gligorov J (2005) Docetaxel in the treatment of breast cancer: current experience and future prospects. Expert Rev Anticancer Ther 5: 613–633
Costa SD, von Minckwitz G, Raab G et al (1999) The role of docetaxel (Taxotere) in neoadjuvant chemotherapy of breast cancer. Semin Oncol 26:24–31
Goble S, Bear HD (2003) Emerging role of taxanes in adjuvant and neoadjuvant therapy for breast cancer: the potential and the questions. Surg Clin North Am 83:943–971
Hutcheon AW, Heys SD, Sarkar TK (2003) Neoadjuvant docetaxel in locally advanced breast cancer. Breast Cancer Res Treat 79[Suppl 1]:S19–24
Bear HD, Anderson S, Brown A et al (2003) The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 21:4165–4174
Bear HD, Anderson S, Smith RE et al (2006) Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 24:2019–2027
Smith IC, Heys SD, Hutcheon AW et al (2002) Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol 20:1456–1466
von Minckwitz G, Raab G, Caputo A et al (2005) Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol 23:2676–2685
Nabholtz JM, Senn HJ, Bezwoda WR et al (1999) Prospective randomized trial of docetaxel versus mitomycin plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline-containing chemotherapy. 304 Study Group. J Clin Oncol 17:1413–1424
Nabholtz JM, Falkson C, Campos D et al (2003) Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic breast cancer: results of a randomized, multicenter, phase III trial. J Clin Oncol 21:968–975
Estevez LG, Cuevas JM, Anton A et al (2003) Weekly docetaxel as neoadjuvant chemotherapy for stage II and III breast cancer: efficacy and correlation with biological markers in a phase II, multicenter study. Clin Cancer Res 9:686–692
Green MC, Buzdar AU, Smith T et al (2005) Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol 23:5983–5992
(1999) Common Toxicity Criteria, Version 2.0. National Cancer Institute, Cancer Therapy Evaluation Program, Bethesda, MD, pp 1–27
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Ogston KN, Miller ID, Payne S et al (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12:320–327
Durbecq V, Paesmans M, Cardoso F et al (2004) Topoisomerase-II alpha expression as a predictive marker in a population of advanced breast cancer patients randomly treated either with single-agent doxorubicin or single-agent docetaxel. Mol Cancer Ther 3:1207–1214
Jarvinen TA, Liu ET (2003) HER-2/neu and topoisomerase IIalpha in breast cancer. Breast Cancer Res Treat 78:299–311
Barrett-Lee PJ (2005) Growth factor signalling in clinical breast cancer and its impact on response to conventional therapies: a review of chemotherapy. Endocr Relat Cancer 12[Suppl 1]:S125–133
Tanner M, Isola J, Wiklund T et al (2006) Topoisomerase IIalpha gene amplification predicts favorable treatment response to tailored and dose-escalated anthracycline-based adjuvant chemotherapy in HER-2/neu-amplified breast cancer: Scandinavian Breast Group Trial 9401. J Clin Oncol24:2428–2436
Arriola E, Moreno A, Varela M et al (2006) Predictive value of HER-2 and Topoisomerase IIalpha in response to primary doxorubicin in breast cancer. Eur J Cancer 42:2954–2960
Manna Edel F, Teixeira LC, Alvarenga M (2006) Association between immunohistochemical expression of topoisomerase IIalpha, HER2 and hormone receptors and response to primary chemotherapy in breast cancer. Tumori 92:222–229
Villman K, Sjostrom J, Heikkila R et al (2006) TOP2A and HER2 gene amplification as predictors of response to anthracycline treatment in breast cancer. Acta Oncol 45:590–596
Rody A, Karn T, Gatje R et al (2006) Gene expression profiling of breast cancer patients treated with docetaxel, doxorubicin, and cyclophosphamide within the GEPARTRIO trial: HER-2, but not topoisomerase II alpha and microtubuleassociated protein tau, is highly predictive of tumor response. Breast 16:86–93
Zaffaroni N, Pennati M, Daidone MG (2005) Survivin as a target for new anticancer interventions. J Cell Mol Med 9:360–372
Tanaka K, Iwamoto S, Gon G et al (2000) Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res 6:127–134
Salz W, Eisenberg D, Plescia J et al (2005) A survivin gene signature predicts aggressive tumor behavior. Cancer Res 65:3531–3534
Span PN, Sweep FC, Wiegerinck ET et al (2004) Survivin is an independent prognostic marker for risk stratification of breast cancer patients. Clin Chem 50:1986–1993
Ryan BM, Konecny GE, Kahlert S et al (2006) Survivin expression in breast cancer predicts clinical outcome and is associated with HER2, VEGF, urokinase plasminogen activator and PAI-1. Ann Oncol 17:597–604
Kennedy SM, O’Driscoll L, Purcell R et al (2003) Prognostic importance of survivin in breast cancer. Br J Cancer 88:1077–1083
O’Driscoll L, Linehan R, M Kennedy S et al (2003) Lack of prognostic significance of survivin, survivin-deltaEx3, survivin-2B, galectin-3, bag-1, bax-alpha and MRP-1 mRNAs in breast cancer. Cancer Lett 201:225–236
Alkarain A, Slingerland J (2004) Deregulation of p27 by oncogenic signaling and its prognostic significance in breast cancer. Breast Cancer Res 6:13–21
Clarke RB (2003) p27KIP1 phosphorylation by PKB/Akt leads to poor breast cancer prognosis. Breast Cancer Res 5:162–163
Barbareschi M (1999) p27 Expression, a cyclin dependent kinase inhibitor in breast carcinoma. Adv Clin Pathol 3:119–127
Barnes A, Pinder SE, Bell JA et al (2003) Expression of p27kip1 in breast cancer and its prognostic significance. J Pathol 201:451–459
Colozza M, Azambuja E, Cardoso F et al (2005) Proliferative markers as prognostic and predictive tools in early breast cancer: where are we now? Ann Oncol 16:1723–1739
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Estevez, L.G., Fortes, J.L., Adrover, E. et al. Doxorubicin and cyclophosphamide followed by weekly docetaxel as neoadjuvant treatment of early breast cancer: analysis of biological markers in a GEICAM phase II study. Clin Transl Oncol 11, 54–59 (2009). https://doi.org/10.1007/s12094-009-0311-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-009-0311-4