Abstract
In order to unravel the biochemical pathways and understand the molecular mechanisms involved in leaf senescence, suppression subtractive hybridization (SSH) was used to generate a cDNA library enriched for transcripts differentially expressed in developmental senescence cotyledons of upland cotton. After differential screening by membrane-based hybridization and subsequent confirmation by reverse Northern blot analysis, selected 678 clones were sequenced and analyzed. Sequencing of these cDNA fragments reveals that 216 of expressed sequence tags (ESTs) represented unique genes. Of these 216 cDNAs, 151 clones (69.9%) show significant homologies to previously known genes, while the remaining 65 do not match any known sequences. 151 unique ESTs are assigned to twelve different categories based on their putative functions generated by BLAST analysis. These SAG-encoded proteins are likely to participate in macromolecule degradation, nutrient recycling, detoxification of oxidative metabolites, and signaling and regulatory events. The expression pattern of selection of genes was confirmed using northern hybridization. Northern hybridization confirmed several distinct patterns, from expression at a very early stage to the terminal phase of the senescence syndrome. Clones encoding proteases and proteins involved in macromolecule degradation and gluconeogenesis, as well as stress-related genes, are up regulated in senescence cotyledons.
Similar content being viewed by others
References
Buchanan-Wollaston V, Earl S, Harrison E, et al. The molecular analysis of leaf senescence — A genomics approach. Plant Biotech J, 2003, 1(1): 3–22
Gan S, Amasino, R M. Making sense of senescence. Molecular genetic regulation and manipulation of leaf senescence. Plant Physiol, 1997, 113(2): 313–319
Guo Y, Cai Z, Gan S. Transcriptome of Arabidopsis leaf senescence. Plant Cell Environ, 2004, 27(5): 521–549
Lin J F, Wu S H. Molecular events in senescing Arabidopsis leaves. Plant J, 2004, 39(4): 612–628
Kakani V G, Reddy K R, Zhao D, et al. Senescence and hyperspectral reflectance of cotton leaves exposed to ultraviolet-B radiation and carbon dioxide. Physiol Plant, 2004, 121(2): 250–257
Shen F F, Yu S X, Han X L, et al. Cloning and characterization of a gene encoding cysteine proteases from senescent leaves of Gossypium hirsutum, Chin Sci Bull, 2004, 49(23): 2601–2607
Wright P R. Premature senescence of cotton (Gossypium hirsutum L.)—Predominantly a potassium disorder caused by an imbalance of source and sink. Plant Soil, 1999, 211(2): 231–239
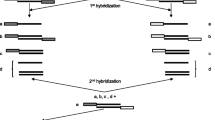
Diatchenko L, Lau Y F, Campbell A P, et al. Suppression subtractive hybridization: A method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci USA, 1996, 93(12): 6025–6030
Arnon D I. Copper enzymes in isolated chloroplasts: Polyphenoloxidase in Betavulgaris. Plant Physiol, 1949, 24(1): 1–15
Gut H, Matile P, Apparent induction of key enzymes of the glyoxylic acid cycle in senescent barley leaves. Planta, 1988, 176(4): 548–550
Wanner L, Keller F, Matile P. Metabolism of radiolabelled galactolipids in senescent barley leaves. Plant Sci, 1991, 78(2): 199–206
Pontier D, Gan S, Amasino R M, et al. Markers for hypersensitive response and senescence show distinct patterns of expression. Plant Mol Biol, 1999, 39(6): 1243–1255
Miller J D, Arteca R N, Pell E J. Senescence-associated gene expression during ozone-induced leaf senescence in Arabidopsis. Plant Physiol, 1999, 120(4): 1015–1024
John C F, Morris K, Jordan B R, et al. Ultraviolet-B exposure leads to upregulation of senescence-associated genes in Arabidopsis thaliana. J Exp Bot, 2001, 52(359): 1367–1373
Ishida H, Anzawa D, Kokubun N, et al. Direct evidence for non-enzymatic fragmentation of chloroplastic glutamine synthetase by a reactive oxygen species. Plant Cell Environ, 2002, 25(5): 625–631
Hortensteiner S, Feller U, Nitrogen metabolism and remobilization during senescence. J Exp Bot, 2002, 53(370): 927–937
Guerinot M L, Yi Y, Iron: Nutritious, noxious, and not readily available. Plant Physiol, 1994, 104(3): 815–820
Bhalerao R, Keskitalo J, Sterky F, et al. Gene expression in autumn leaves. Plant Physiol, 2003, 131(2): 430–442
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Shen, F., Yu, S., Xie, Q. et al. Identification of genes associated with cotyledon senescence in upland cotton. CHINESE SCI BULL 51, 1085–1094 (2006). https://doi.org/10.1007/s11434-006-1085-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11434-006-1085-5