Abstract
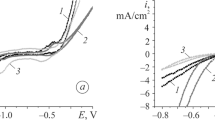
Electrochemical corrosion and oxidation resistances of Zr60Ni21Al19 amorphous alloy were studied. The ternary amorphous alloy exhibits greater positive potential than its crystalline counterpart and 0Cr19Ni9Ti stainless steel. Its weight loss result measured in 2 mol/L HCl solution is in agreement with the potentiodynamic curve. But there is no obvious difference in the oxidation resistances between Zr60Ni21Al19 amorphous and its crystalline alloys. They both exhibit high oxidation resistance.
Similar content being viewed by others
References
Wang W H, Dong C, Shek C H. Bulk metallic glasses. Mater Sci Eng R, 2004, 44: 45–89
Inoue A. Slowly-cooled bulk amorphous alloys. Mater Sci Forum, 1995, 179–181: 691–700
Johnson W L. Bulk glass-forming metallic alloys science and technology. MRS Bull, 1999, 10: 42–45
Perkr A, Johnson W L. A highly processing metallic glass: Zr41.2Ti13.8Cu12.5Ni10Be22.5. Appl Phys Lett, 1993, 63: 2342–2344
Wang D, Tan H, Li Y. Multiple maxima of GFA in three adjacent eutectics in Zr-Cu-Al alloy system-A metallographic way to pinpoint the best glass forming alloys. Acta Mater, 2005, 53: 2969–2979
Kumagai T, Nikkuni D, Hara S, et al. Development of interatomic potential for Zr-Ni amorphous systems. Mater Trans, 2007, 48: 1313–1321
Matsubara E, Ichitsubo T, Saida J, et al. Structural study of Zr-based metallic glasses. J Alloys Compd, 2007, 434–435: 119–120
Jing Q, Zhang Y, Wang D, et al. A study of glass forming ability in ZrNiAl alloys. Mater Sci Eng A, 2006, 441: 106–111
Koster U, Triwikantoro. Oxidation of amorphous and nanocrystalline Zr-Cu-Ni-Al alloys. Mater Sci Forum, 2001, 360–362: 29–36
Kimura H M, Asami K, Inoue A, et al. The oxidation of amorphous Zr-base binary alloys in air. Corro Sci, 1993, 35: 909–915
Huang D Y, Zhao X G, Zhang T, et al. Air oxidation kinetics study of Zr58Nb3Cu16Ni13Al10 bulk metallic glass. Defect Diffu Forum, 2009, 283–286: 209–213
Koster U, Schunemann U. Rapidly Solidified Alloys. In: Liebermann H H, ed. New York: Marcel Dekker Inc., 1993
Kim C W, Jeong H G, Lee D B. Oxidation of Zr65Al10Ni10Cu15 bulk metallic glass. Mater Lett, 2008, 62: 584–586
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jing, Q., Zhang, B., Zhang, J. et al. Electrochemical corrosion and oxidation resistances of Zr60Ni21Al19 bulk amorphous alloys. Sci. China Phys. Mech. Astron. 53, 2223–2226 (2010). https://doi.org/10.1007/s11433-010-4185-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11433-010-4185-9