Abstract
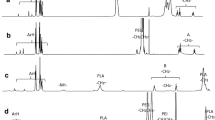
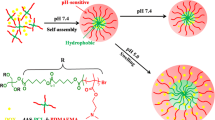
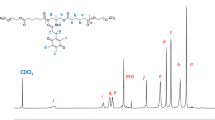
A novel type of bioreducible amphiphilic multiarm hyperbranched copolymer (H40-star-PLA-SS-PEG) based on Boltorn® H40 core, poly(l-lactide) (PLA) inner-shell, and poly(ethylene glycol) (PEG) outer-shell with disulfide-linkages between the hydrophobic and hydrophilic moieties was developed as unimolecular micelles for controlled drug release triggered by reduction. The obtained H40-star-PLA-SS-PEG was characterized in detail by nuclear magnetic resonance (NMR), Fourier transform infrared (FTIR), gel permeation chromatography (GPC), differential scanning calorimeter (DSC), and thermal gravimetric analysis (TGA). Transmission electron microscopy (TEM) and dynamic light scattering (DLS) analyses suggested that H40-star-PLA-SS-PEG formed stable unimolecular micelles in aqueous solution with an average diameter of 19 nm. Interestingly, these micelles aggregated into large particles rapidly in response to 10 mM dithiothreitol (DTT), most likely due to shedding of the hydrophilic PEG outer-shell through reductive cleavage of the disulfide bonds. As a hydrophobic anticancer model drug, doxorubicin (DOX) was encapsulated into these reductive unimolecular micelles. In vitro release studies revealed that under the reduction-stimulus, the detachment of PEG outer-shell in DOX-loaded micelles resulted in a rapid drug release. Flow cytometry and confocal laser scanning microscopy (CLSM) measurements indicated that these DOX-loaded micelles were easily internalized by living cells. Methyl tetrazolium (MTT) assay demonstrated a markedly enhanced drug efficacy of DOX-loaded H40-star-PLA-SS-PEG micelles as compared to free DOX. All of these results show that these bioreducible unimolecular micelles are promising carriers for the triggered intracellular delivery of hydrophobic anticancer drugs.
Similar content being viewed by others
References
Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discovery, 2003, 2: 347–360
Lukyanov AN, Torchilin VP. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Delivery Rev, 2004, 56: 1273–1289
Kabanov AV. Polymer genomics: An insight into pharmacology and toxicology of nanomedicines. Adv Drug Delivery Rev, 2006, 58: 1597–1621
Zhang L, Eisenberg A. Formation of crew-cut aggregates of various morphologies from amphiphilic block copolymers in solution. Polym Adv Technol, 1998, 9: 677–699
van Hest JCM, Delnoye DAP, Baars MWPL, van Genderen MHP, Meijer EW. Polystyrene-dendrimer amphiphilic block copolymers with a generation-dependent aggregation. Science, 1995, 268: 1592–1595
Cho BK, Jain A, Nieberle J, Mahajan S, Wiesner U, Gruner SM, Türk S, Räder HJ. Synthesis and self-assembly of amphiphilic dendrimers based on aliphatic polyether-type dendritic cores. Macromolecules, 2004, 37: 4227–4234
Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv Drug Delivery Rev, 2001, 47: 113–131
Torchilin VP. Multifunctional nanocarriers. Adv Drug Delivery Rev, 2006, 58: 1532–1555
Wang F, Bronich TK, Kabanov AV, Rauh RD, Roovers J. Synthesis and evaluation of a star amphiphilic block copolymer from poly-(ɛ-caprolactone) and poly(ethylene glycol) as a potential drug delivery carrier. Bioconjugate Chem, 2005, 16: 397–405
Lawrence MJ. Surfactant systems: their use in drug delivery. Chem Soc Rev, 1994, 23: 417–424
Kainthan RK, Brooks DE. Unimolecular micelles based on hydrophobically derivatized hyperbranched polyglycerols: biodistribution studies. Bioconjugate Chem, 2008, 19: 2231–2238
Kontoyianni C, Sideratou Z, Theodossiou T, Tziveleka L, Tsiourvas D, Paleos CM. A novel micellar PEGylated hyperbranched polyester as a prospective drug delivery system for paclitaxel. Macromol Biosci, 2008, 8: 871–881
Kitajyo Y, Kinugawa Y, Tamaki M, Kaga H, Kaneko N, Satoh T, Kakuchi T. Synthesis, characterization, and functionalization of hyperbranched poly(3,4-epoxycyclohexanemethanol). Macromolecules, 2007, 40: 9313–9321
Ternat C, Ouali L, Sommer H, Fieber W, Velazco MI, Plummer CJG, Kreutzer G, Klok HA, Månson JE, Herrmann A. Investigation of the release of bioactive volatiles from amphiphilic multiarm star-block copolymers by thermogravimetry and dynamic headspace analysis. Macromolecules, 2008, 41: 7079–7089
Kreutzer G, Ternat C, Nguyen TQ, Plummer CJG, Månson JAE, Castelletto V, Hamley IW, Sun F, Sheiko SS, Herrmann A, Ouali L, Sommer H, Fieber W, Velazco MI, Klok HA. Water-soluble, unimolecular containers based on amphiphilic multiarm star block copolymers. Macromolecules, 2006, 39: 4507–4516
Du WJ, Xu ZQ, Nyström AM, Zhang K, Leonard JR, Wooley KL. 19F- and fluorescently labeled micelles as nanoscopic assemblies for chemotherapeutic delivery. Bioconjugate Chem, 2008, 19: 2492–2498
Zou JH, Shi WF, Wang J, Bo J. Encapsulation and controlled release of a hydrophobic drug using a novel nanoparticle-forming hyperbranched polyester. Macromol Biosci, 2005, 5: 662–668
Xu J, Luo S Z, Shi WF, Liu SY. Two-stage collapse of unimolecular micelles with double thermoresponsive coronas. Langmuir, 2006, 22: 989–997
Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S. Folate-conjugated amphiphilic hyperbranched block copolymers based on Boltorn® H40, poly(l-lactide) and poly(ethylene glycol) for tumor targeted drug delivery. Biomaterials, 2009, 30: 3009–3019
Chen S, Zhang XZ, Cheng SX, Zhuo RX, Gu ZW. Functionalized amphiphilic hyperbranched polymers for targeted drug delivery. Biomacromolecules, 2008, 9: 2578–2585
Radowski MR, Shukla A, von Berlepsch H, Böttcher C, Pickaert G, Rehage H, Haag R. Supramolecular aggregates of dendritic multishell architectures as universal nanocarriers. Angew Chem Int Ed, 2007, 46: 1265–1269
Tian HY, Chen XS, Lin H, Deng C, Zhang PB, Wei Y, Jing XB. Micellization and reversible pH-sensitive phase transfer of the hyperbranched multiarm PEI-PBLG copolymer. Chem Eur J, 2006, 12: 4305–4312
Zhou YF, Yan DY. Supramolecular self-assembly of amphiphilic hyperbranched polymers at all scales and dimensions: Progress, characteristics and perspectives. Chem Commun, 2009, 1172-1178
Liu H, Chen Y, Shen Z. Thermoresponsive hyperbranched polyethylenimines with isobutyramide functional groups. J Polym Sci Part A: Polym Chem, 2007, 45: 1177–1184
Haba Y, Harada A, Takagishi T, Kono K. Rendering poly(amidoamine) or poly(propylenimine) dendrimers temperature sensitive. J Am Chem Soc, 2004, 126: 12760–12761
Tai H, Wang W, Alexander C, Shakesheff KM, Howdle SM. Thermal-responsive and photocrosslinkable hyperbranched polymers synthesised by deactivation enhanced ATRP and RAFT polymerizations. J Controlled Release, 2008, 132: e48–e50
Carter S, Hunt B, Rimmer S. Highly branched poly(N-isopropy-lacrylamide)s with imidazole end groups prepared by radical polymerization in the presence of a styryl monomer containing a dithioester group. Macromolecules, 2005, 38: 4595–4603
Jia ZF, Chen H, Zhu XY, Yan DY. Backbone-thermoresponsive hyperbranched polyethers. J Am Chem Soc, 2006, 128: 8144–8145
Calderón M, Quadir MA, Sharma SK, Haag R. Dendritic polyglycerols for biomedical applications. Adv Mater, 2010, 22: 190–218
Chen J, Wu C, Oupický D. Bioreducible hyperbranched poly(amido amine)s for gene delivery. Biomacromolecules, 2009, 10: 2921–2927
You YZ, Hong CY, Pan CY. Facile one-pot approach for preparing dually responsive core-shell nanostructure. Macromolecules, 2009, 42: 573–575
Lin C, Blaauboer CJ, Timoneda MM, Lok MC, van Steenbergen M, Hennink WE, Zhong ZY, Feijen J, Engbersen JF. Bioreducible poly(amido amine)s with oligoamine side chains: Synthesis, characterization, and structural effects on gene delivery. J Controlled Release, 2008, 126: 166–174
Christensen LV, Chang CW, Kim WJ, Kim SW, Zhong ZY, Lin C, Engbersen JF, Feijen J. Reducible poly(amido ethylenimine)s designed for triggered intracellular gene delivery. Bioconjugate Chem, 2006, 17: 1233–1240
Meng FH, Hennink WE, Zhong ZY. Reduction sensitive polymers and bioconjugates for biomedical applications. Biomaterials, 2009, 30: 2180–2198
Saito G, Swanson JA, Lee KD. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities. Adv Drug Delivery Rev, 2003, 55: 199–215
Russo A, DeGraft W, Friedman N, Mitchell JB. Selective modulation of glutathione levels in human normal versus tumor cells and subsequent differential response to chemotherapy drugs. Cancer Res, 1986, 46: 2845–2848
Ryu JH, Roy R, Ventura J, Thayumanavan S. Redox-sensitive disassembly of amphiphilic copolymer based micelles. Langmuir, 2010, 26: 7086–7092
Tang LY, Wang YC, Li Y, Du JZ, Wang J. Shell-detachable micelles based on disulfide-linked block copolymer as potential carrier for intracellular drug delivery. Bioconjugate Chem, 2009, 20: 1095–1099
Sun Y, Yan XL, Yuan TM, Liang J, Fan YJ, Gu ZW, Zhang XD. Disassemblable micelles based on reduction-degradable amphiphilic graft copolymers for intracellular delivery of doxorubicin. Biomaterials, 2010, 31: 7124–7131
Sun HL, Guo BN, Li XQ, Cheng R, Meng FH, Liu HY, Zhong ZY. Shell-sheddable micelles based on dextran-SS-poly(ɛ-caprolactone) diblock copolymer for efficient intracellular release of doxorubicin. Biomacromolecules, 2010, 11: 848–854
Li YL, Zhu L, Liu ZZ, Cheng R, Meng FH, Cui JH, Ji SJ, Zhong ZY. Reversibly stabilized multifunctional dextran nanoparticles efficiently deliver doxorubicin into the nuclei of cancer cells. Angew Chem Int Ed, 2009, 48: 9914–9918
Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S. Amphiphilic multi-arm-block copolymer conjugated with doxorubicin via pH-sensitive hydrazone bond for tumor-targeted drug delivery. Biomaterials, 2009, 30: 5757–5766
Yang X, Grailer JJ, Pilla S, Steeber DA, Gong S. Tumor-targeting, pH-tesponsive, and stable unimolecular micelles as drug nanocarriers for targeted cancer therapy. Bioconjugate Chem, 2010, 21: 496–504
Liu JY, Huang W, Pang Y, Zhu XY, Zhou YF, Yan DY. Self-assembled micelles from an amphiphilic hyperbranched copolymer with poly-phosphate arms for drug delivery. Langmuir, 2010, 26: 10585–10592
Fang JH, Kita H, Okamoto KI. Hyperbranched polyimides for gas separation applications. 1. Synthesis and characterization. Macromolecules, 2000, 33: 4639–4646
Schmalenberg KE, Frauchiger L, Nikkhouy-Albers L, Uhrich KE. Cytotoxocity of a unimolecular polymeric micelle and its degradation products. Biomacromolecules, 2001, 2: 851–855
Akinc A, Anderson DG, Lynn DM, Langer R. Synthesis of poly(ɛ-amino ester)s optimized for highly effective gene delivery. Bioconjugate Chem, 2003, 14: 979–988
Gewirtz DA. A critical evaluation of the mechanisms of action pro-posed for the antitumor effects of the anthracycline anti-biotics, adriamycin and daunorubicin. Biochem Pharmacol, 1999, 57: 727–741
Pang Y, Zhu Q, Liu JY, Wu JL, Wang RB, Chen SY, Zhu XY, Yan DY, Huang W, Zhu BS. Design and synthesis of cationic drug carriers based on hyperbranched poly(amine-ester)s. Biomacromolecules, 2010, 11: 575–582
Liu JY, Huang W, Pang Y, Zhu XY, Zhou YF, Yan DY. Hyper-branched polyphosphates for drug delivery application: Design, synthesis, and in vitro evaluation. Biomacromolecules, 2010, 11: 1564–1570
Sun XY, Zhou YF, Yan DY. Drug release property of a pH-reponsive double-hydrophilic hyperbranched graft coploymer. Sci China Ser B-Chem, 2009, 51(10): 1703–1710
Liu JY, Pang Y, Huang W, Zhu XY, Zhou YF, Yan DY. Self-assembly of phospholipid-analogous hyperbranched polymers nanomicelles for drug delivery. Biomaterials, 2010, 31: 1334–1341
Liu JY, Huang W, Pang Y, Zhu XY, Zhou YF, Yan DY. The in vitro biocompatibility of self-assembled hyperbranched copolyphosphate nanocarriers. Biomaterials, 2010, 31: 5643–5651
Shuai X, Ai H, Nasongkla N, Kim S, Gao J. Micellar carriers based on block copolymers of poly(ɛ-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J Controlled Release, 2004, 98: 415–426
Soppimath KS, Liu LH, Seow WY, Liu SQ, Powell R, Chan P, Yang YY. Multifunctional core/shell nanoparticles self-assembled from pH-induced thermosensitive polymers for targeted intracellular anticancer drug delivery. Adv Funct Mater, 2007, 17: 355–362
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Pang, Y., Liu, J., Su, Y. et al. Bioreducible unimolecular micelles based on amphiphilic multiarm hyperbranched copolymers for triggered drug release. Sci. China Chem. 53, 2497–2508 (2010). https://doi.org/10.1007/s11426-010-4163-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-010-4163-0