Abstract
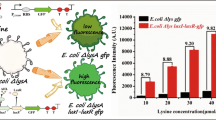
As an essential amino acid, lysine is an important component of animal and human diets and its bioavailability can depend on a variety of factors. Therefore, an accurate pre-determination of bioavailable lysine in foods and feeds is important. In this study a whole cell fluorescent biosensor for the quantification of lysine in protein sources was constructed. A gene encoding for green fluorescent protein (GFPmut3) was introduced into an E. coli lysine auxotroph genome as a part of a mini-Tn5-Km transposon. The location of the transposon was determined and the growth kinetics of the newly constructed biosensor were examined. The transposon disrupted the ybhM gene, which encodes for the synthesis of a protein with an unknown function. No effect of the transposon’s location in the genome or the expression of gfp on bacterial growth rates was observed. Based on the fluorescence emitted by GFPmut3, a standard curve after 6-h growth of the strain was generated. A correlation coefficient of 0.95 was observed when the fluorescence method was compared to the conventional optical density (OD) growth-based lysine assay. Using the newly developed lysine fluorescent whole cell sensor we determined the total lysine in casein acid hydrolyzate (7.13 ± 0.34%). When lysine added to 12 μg/ml and 30 μg/ml of casein acid hydrolyzate was quantified, recoveries of 97 ± 1.65% and 103 ± 4.66% respectively were detected. The results suggest that the microbial assay using GFP fluorescence represents a promising alternative method for the potential estimation of lysine in protein sources.



Similar content being viewed by others
References
Albano CR, Randers-Eichhorn L, Bentley WE, Rao G (1998) Green fluorescent protein as a real time quantitative reporter of heterologous protein production. Biotechnol Progr 14:351–354
Andersen JB, Sternberg C, Poulsen LK, Petersen-Bjorn S, Givskov M, Mølin S (1998) New unstable variants of green fluorescent protein for studies of transient gene expression in bacteria. Appl Environ Microbiol 64:2240–2246
Anderson-Hafermann CJ, Zhang Y, Parsons CM (1993) Effects of processing on the nutritional quality of canola meal. Poult Sci 72:326–333
Bae Y-S, Knudsen GR (2000) Cotransformation of Trichoderma harzianum with a β-glucoronidase and green fluorescent protein genes provides a useful tool for minitoring fungal growth and activity in natural soils. Appl Environ Microbiol 66:810–815
Burlage RS (1997) Emerging technologies: bioreporters, biosensors, and microprobes. In: Hurst CJ, Knudsen GR, McInerny MJ, Stetzenbach LD, Walter MV (eds) Manual of environmental microbiology. ASM Press, Washington, DC, pp 115–123
Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC (1994) Green fluorescent protein as a marker for gene expression. Science 263:802–805
Chalova V, Woodward CL, Ricke SC (2006) Application of an Escherichia coli green fluorescent protein – based biosensor under nonsterile conditions and autofluorescence background. Lett Appl Microbiol 42:265–270
Cody CW, Prasher DC, Westler WM, Prendergast FG, Ward WW (1993) Chemical structure of the hexapeptide chromophore of the Aequorea green-fluorescent protein. Biochemistry 32:1212–1218
Cork LC, Clarkson TB, Jacoby RO, Gaertner DJ, Leary SL, Linn JM, Pakes SP, Ringler DH, Strandberg JD, Swindle MM (1997) The cost of animal research: origins and options. Science 276:758–759
Cormack P, Valdivia R, Falkow S (1996) FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33–38
De Lorenzo V, Timmis KN (1994) Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10- derived minitransposons. Methods Enzymol 235:386–405
De Lorenzo V, Herrero M, Jakubzik U, Timmis KN (1990) Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing and chromosomal insertion of cloned DNA in gram-negative eubacteria J Bacteriol 172:6568–6572
D’Mello JPF (2003) Response of growing poultry to amino acids. In: D’Mello JPF (ed) Amino acids in animal nutrition, CABI publishing, Wallingford, pp 237–263
Erickson AM, Zabala -Díaz IB, Ricke SC (1999) Antibiotic amendment for suppression of indigenous microflora in feed sources for an Escherichia coli auxotroph lysine assay. J Appl Microbiol 87:125–130
Erickson AM, Zabala Díaz IB, Kwon YM, Ricke SC (2000) A bioluminescent Escherichia coli auxotroph for use in an in vitro lysine availability assay. J Microbiol Methods 40:207–212
Erickson AM, Li X, Zabala-Díaz IB, Ricke SC (2002) Potential for measurement of lysine bioavailability in poultry feeds by rapid microbiological assays – A review. J Rapid Methods Auto Micro 10:1–8
Errampalli D, Leung K, Cassidy MB, Kostrzynska M, Blears M, Lee H, Trevors JT (1999) Application of the green fluorescent protein as a molecular marker in environmental microorganisms. J Microbiol Methods 35:187–199
Fratamico PM, Deng MY, Strobaugh TP, Palumbo SA (1997) Construction and characterization of Escherichia coli O157:H7 strains expressing firefly luciferase and green fluorescent protein and there use in survival studies. J Food Prot 60:1167–1173
Guido NJ, Wang X, Adalsteinsson D, McMillen D, Hasty J, Cantor CR, Elston TC, Collins JJ (2006) A bottom-up approach to gene regulation. Nature 439: 856–860
Guthneck BT, Bennett BA, Schweigert BS (1953) Utilization of amino acids from foods by the rat. II. Lysine J Nutr 49:289–294
Hankes LV, Riesen WH, Henderson LM, Elvehjem CA (1948) Liberation of amino acids from raw and heated casein by acid and enzyme hydrolysis. J Biol Chem 176:467–476
Herrero M, de Lorenzo V, Timmis KN (1990) Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol 172:6557–6567
Johnson ML, Parsons CM, Fahey Jr GC, Merchen NR, Aldrich CG (1998) Effects of species raw material source, ash content, and processing temperature on amino acid digestibility of animal by-product meals by cecectomized roosters and ileally cannulated dogs. J Anim Sci 76:1112–1122
Kwon YM, Ricke SC (2000) Efficient amplification of multiple transposon-flanking sequences. J Microbiol Methods 41:195–199
Kwon YM, Kubena LF, Nisbet DJ, Ricke SC (2002) Functional screening of bacterial genome for virulence genes by transposon footprinting. Methods Enzymol 358:141–152
Leveau JHJ, Lindow SE (2001) Predictive and interpretive simulation of green fluorescent protein expression in reporter bacteria. J Bacteriol 183:6752–6762
Li X, Ricke SC (2003) Generation of an Escherichia coli lysA targeted deletion mutant by double cross-over recombination for potential use in a bacterial growth-based lysine assay. Lett Appl Microbiol 37:458–462
Li X, Erickson AM, Ricke SC (1999) Comparison of minimal media and inoculum concentration to decrease the lysine growth assay response time of an Escherichia coli lysine auxotroph mutant. J Rapid Methods Autom Microbiol 7:279–290
Li X, Erickson AM, Ricke SC (2000) Agitation during incubation reduces the time required for a lysine microbiological growth assay using an Escherichia coli auxotrophic mutant. J Rapid Methods Autom Microbiol 8:83–94
McMahan JR, Shell EE (1944) The microbiological determination of amino acids: I. Valine and arginine. J Biol Chem 152:83–95
National Research Council (1994) Nutrient requirements of poultry. National Academy Press, Washington, DC, pp 61–79
Nordheim JP, Coon CN (1984) A comparison of four methods for determining available lysine in animal protein meals. Poult Sci 63:1040–1051
Oscar TP (2003) Comparison of predictive models for growth of parent and green fluorescent protein-producing strains of Salmonella. J Food Prot 66:200–207
Oscar TP, Dulal K, Boucaud D (2006) Transformation of Escherichia coli K-12 with a high-copy plasmid encoding the green fluorescent protein reduces growth: implications for predictive microbiology. J Food Prot 69:276–281
Payne JW, Bell G, Higgins CF (1977) The use of Escherichia coli Lys− auxotroph to assay nutritionally available lysine in biological materials. J Appl Bacteriol 42:165–177
Pitcher DG, Saunders NA, Owen RJ (1989) Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol 8:151–156
Prachaiyo P, McLandsborough LA (2000) A microscopic method to visualize Escherichia coli interaction with beef muscle. J Food Prot 63:427–433
Ricke SC, Schaefer DM (1991) Growth inhibition of the rumen bacterium Selenomonas ruminantium by ammonium salts. Appl Microbiol Biotechnol 36:394–399
Sambrook J, Frish EF, Maniatis T (1989) Molecular Cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Seabury CM, Derr JN (2003) Identification of a novel ovine PrP polymorphism and scrapie-resistant genotypes for St. Croix White and related composite breed. Cytogenet Genome Res 102:85–88
Sibbald IR, Wolynetz MS (1985) The bioavailability of supplementary lysine and its effect on the energy and nitrogen excretion of adult cockerels fed diets diluted with cellulose. Poult Sci 64:1972–1975
Shetty RS, Ramanathan S, Badr IH, Wolford JL, Daunert S (1999) Green fluorescent protein in the design of a living biosensing system for l-arabinose. Anal Chem 71:763–768
Shimomura O, Johnson FH, Saiga Y (1962) Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol 59:223–239
Tsien R (1998) The green fluorescent protein. Annu Rev Biochem 67:509–544
Udenfriend S (1962) Principles of fluorescence. In: Udenfriend S (ed) Fluorescence assay in biology and medicine, vol 1. Academic Press, New York, NY, pp 5–35
Vialette M, Jandos-Rudnik AM, Guyard C, Legeay O, Pinon A, Lange M (2004) Validating the use of green fluorescent-marked Escherichia coli O157:H7 for assessing the organism behaviour in foods. J Appl Microbiol 96:1097–1104
Zabala Díaz IB, Erickson AM, Ricke SC (1999) Growth response and recovery in selective media of a lysine auxotroph Escherichia coli for a rapid microbiological assay. J Rapid Methods Autom Microbiol 7:263–278
Zabala Díaz IB, Ricke SC (2003) Quantitative detection of crystalline lysine supplementation in poultry feeds using a rapid bacterial bioluminescence assay. Appl Microbiol Biotechnol 62:268–273
Acknowledgements
This research was supported by Hatch grant H8311 administered by the Texas Agricultural Experiment Station and Texas Advanced Technology Program, grant #: 000517-0220-2001 (Texas Higher Education Board, Austin, TX, USA). The authors would like to express their thanks to Julia Sonka (Center for Food Safety and Microbiology, IFSE, University of Arkansas) for her assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chalova, V.I., Zabala-Díaz, I.B., Woodward, C.L. et al. Development of a whole cell green fluorescent sensor for lysine quantification. World J Microbiol Biotechnol 24, 353–359 (2008). https://doi.org/10.1007/s11274-007-9479-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-007-9479-3