Abstract
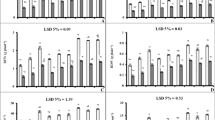
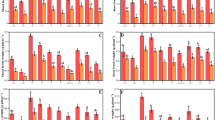
Antimony (Sb) pollution in the downstream farmland soil of the Sb mine area has been of a great environmental concern to the local residents. However, effects of Sb on the growth and physiology of crops are still not well known. In the present study, Sb uptake and its effect on growth, antioxidant defense system, and photosynthesis of maize (Zea mays) were investigated. Our results demonstrated that accumulation of Sb in the maize increased with increasing Sb level in the soil. Sb could be easily translocated from root to shoot with a translocation coefficient over 2.05. Plant growth and biomass were reduced due to Sb pollution. Under Sb stress, the activities of peroxidase (POD), superoxide dismutases (SOD), and catalase (CAT) responded differently. The activities of POD and SOD were inhibited when the soil Sb concentration was higher than 50 mg kg−1. CAT activity showed an increasing trend with increasing soil Sb concentration. Chlorophyll synthesis and the maximum photochemical efficiency (F V/F M) were also inhibited significantly under stress of high-level Sb in soil.



Similar content being viewed by others
References
Alan, R. W. (1994). The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Plant Physiology, 144, 307–313.
An, Y. J., & Kim, M. J. (2009). Effect of antimony on the microbial growth and the activities of soil enzymes. Chemosphere, 74, 654–659.
Baroni, F., Boscagli, A., Protano, G., & Riccoboano, F. (2000). Antimony accumulation in Achillea ageratum, lantago lanceolata and Silene vulgaris growing in an old Sb-mining area. Environmental Pollution, 109, 347–352.
Council of the European Communities. (1976). Council Directive 76/464/EEC of 4 May 1976 on pollution caused by certain dangerous substances discharged into the aquatic environment of the Community. Official Journal L, 129, 23–29.
Dan, T. V., KrishnaRaj, S., & Saxena, P. K. (2000). Metal tolerance of scented geranium (Pelargonium sp. ‘Frensham’): Effects of cadmium and nickel on chlorophyll fluorescence kinetics. International Journal of Phytoremediation, 2, 91–94.
Devi, S.R. & Prasad, M.N.V. (1998).Copper toxicity in Ceratophyllum demersum L. (coontail), a free-floating macrophyte: response of antioxidant enzymes and antioxidants. Plant Science, 138, 157–165.
Dietz, K. J., Baier, M., & Kramer, U. (1999). Free radicals and reactive oxygen species as mediators of heavy metal toxicity in plants. In M. N. V. Prasad & J. Hagemeyer (Eds.), Heavy metal stress in plants-from molecules to ecosystems (pp. 73–98). Berlin: Springer-Verlag.
Filella, M., Belzile, N., & Chen, Y. W. (2002). Antimony in the environment: A review focused on natural waters I. Occurrence. Earth Science Review, 57, 125–176.
Giannopolitis, N., & Ries, S. K. (1977). Superoxide dismutase I: Occurrence in higher plants. Plant Physiology, 59, 309–314.
Goel, A., & Sheoran, I. S. (2003). Lipid peroxidation and peroxide-scavenging enzyme in cotton seeds under natural ageing. Biologia Plantarum, 46, 429–434.
Hammel, W., Debus, R., & Steubing, L. (2000). Mobility of antimony in soil and its availability to plants. Chemosphere, 41, 1791–1798.
He, M. C., & Yang, J. R. (1999). Effects of different forms of antimony on rice during the period of germination and growth and antimony concentration in rice tissue. Science of Total Environment, 243–244, 149–155.
He, M. C., Ji, H. B., Zhao, C. Y., Xie, J., Wu, X. M., & Li, Z. F. (2002). Preliminary studies of heavy metal pollution in soil and plant near antimony mine area. Journal of Beijing Normal University (Natural Science), 38, 417–420.
Hozhina, E. I., Khramov, A. A., Gerasimov, P. A., & Kumarkov, A. A. (2001). Uptake of heavy metals, arsenic, and antimony by aquatic plants in the vicinity of ore mining and processing industries. Journal of Geochemical Exploration, 74, 153–162.
International Agency for Research on Cancer (1998). http://www.inchem.org/documents/iarc/vol47/47-11.html.
Kalpana, R., & Madhava, R. K. V. (1995). On the aging mechanism in pigeonpea (Cajanus cajan L.) seed. Seed Science and Technology, 23, 1–9.
Krachler, M., Emons, H., & Zheng, J. (2001). Speciation of antimony for the 21st century: Promises and pitfalls. TrAC Trends in Analytical Chemistry, 20, 79–90.
Luna, C.M., Gonzalez, V.S. & Trippi, V.S. (1994). Oxidative damage caused by excess copper in oat leaves. Plant Cell Physiology, 35, 11–15.
Pratas, J., Prasad, M. N. V., Freitas, H., & Conde, L. (2005). Plants growing in abandoned mines of Portugal are useful for biogeochemical exploration of arsenic, antimony, tungsten and mine reclamation. Journal of Geochemical Exploration, 85, 99–107.
Scandalios, J.G. (1993). Oxygen stress and superoxide dismutase. Plant Physiology, 101, 7–12.
Shotyk, W., Krachler, M., & Chen, B. (2004). Antimony in recent, ombrotrophic peat from Switzerland and Scotland: comparison with natural background values (5,320 to 8,020 14C yr BP) and implications for the global atmospheric Sb cycle. Global Biogeochemical Cycles, 18, GB1016.1–GB1016.13.
Smichowski, P. (2007). Antimony in the environment as a global pollutant: a review on analytical methodologies for its determination in atmospheric aerosols. Talanta, 75, 2–14.
Tewari, R. K., Kumar, P., & Sharma, P. N. (2006). Magnesium deficiency induced oxidative stress and antioxidant responses in mulberry plants. Scientia Horticulturae, 108, 7–14.
United States Environmental Protection Agency (1979). Water related fate of the 129 priority pollutants, vol. 1 (pp. 23–29). Washington, DC: USEPA, EP-440/4-79-029A.
Winston, G.W. (1990). Physiological basis for free radical formation in cells: production and defenses In: R. Alscher & J. Cummings (Eds.), Stress Responses in Plants-Adaptation and Acclimation Mechanisms (pp. 57–58). Wiley Liss: New York.
Zhu, J., Wu, F. C., Deng, Q. J., Shao, S. X., Mo, C. L., Pan, X. L., et al. (2009). Environmental characteristics of water near the Xikuangshan antimony mine, Hunan province. Acta scientiae Circumstantiae, 9, 655–66l.
Acknowledgments
This work was supported by the Knowledge Innovation Program of Chinese Academy of Sciences (KZCX2-YW-335), Program of 100 Distinguished Young Scientists of the Chinese Academy of Sciences, and National Natural Science Foundation of China (40872169). We are grateful to the anonymous reviewers for their valuable comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pan, X., Zhang, D., Chen, X. et al. Antimony Accumulation, Growth Performance, Antioxidant Defense System and Photosynthesis of Zea mays in Response to Antimony Pollution in Soil. Water Air Soil Pollut 215, 517–523 (2011). https://doi.org/10.1007/s11270-010-0496-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0496-8