Abstract
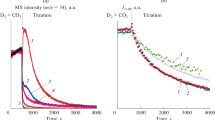
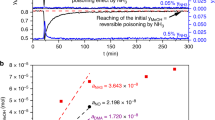
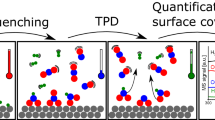
The coverages and surface lifetimes of copper-bound formates on Cu/SiO2 catalysts, and the steady-state rates of reverse water-gas shift and methanol synthesis have been measured simultaneously by mass (MS) and infrared (IR) spectroscopies under a variety of elevated pressure conditions at temperatures between 140 and 160 °C. DCOO lifetimes under steady state catalytic conditions in CO2:D2 atmospheres were measured by 12C–13C isotope transients (SSITKA). The values range from 220 s at 160 °C to 660 s at 140 °C. The catalytic rates of both reverse water gas shift (RWGS) and methanol synthesis are ~100-fold slower than this formate removal rate back to CO2 + 1/2 H2, and thus they do not significantly influence the formate lifetime or coverage at steady state. The formate coverage is instead determined by formate’s rapid production/decomposition equilibrium with gas phase CO2 + H2. The results are consistent with formate being an intermediate in methanol synthesis, but with the rate-controlling step being after formate production (for example, its further hydrogenation to methoxy). A 2–3 fold shorter life time (faster decomposition rate) was observed for formate under reactions conditions, with both D2 and CO2 present, than in pure Ar or D2 + Ar alone. This effect, due in part to the effects of the coadsorbates produced under reaction conditions, illustrates the importance of using in situ techniques in the study of catalytic mechanisms. The carbon which appears in the methanol product spends a longer time on the surface than the formate species, 1.8 times as long at 140 °C. The additional delay on the surface is attributed in part to readsorption of methanol on the catalyst, thus obscuring the mechanistic link between formate and methanol.






Similar content being viewed by others
References
Chinchen GC, Denny PJ, Jennings JR, Spencer MS, Waugh KC (1988) Appl Catal 36:1
Askgaard TS, Nørskov JK, Ovesen CV, Stolze P (1995) J Catal 156:229
Schumacher N, Boisen A, Dahl S, Gokhale AA, Kandoi S, Grabow LC, Dumesic JA, Mavrikakis M, Chorkendorff I (2005) J Catal 229:265
Qi XM, Flytzani-Stephanopoulos M (2004) Ind Eng Chem Res 43:3055
Chorkendorff I, Taylor PA, Rasmussen PB (1992) J Vac Technol A 10:2277
Wachs IE, Madix RJ (1978) J Catal 53:208
Russell JN Jr, Gates SM, Yates JT Jr (1985) Surf Sci 163:516
Ernst K-H, Campbell CT, Moretti G (1992) J Catal 134:66
Gokhale AA, Dumesic JA, Mavrikakis M (2008) J Am Chem Soc 130:1402
Burch R, Golunski SE, Spencer MS (1990) Catal Lett 5:55
Fujitani T, Nakamura I, Uchijima T, Nakamura J (1997) Surf Sci 383:285
Fujitani T, Nakamura I, Ueno S, Uchijima T, Nakamura J (1997) Appl Surf Sci 121:583
Wachs IE, Madix RJ (1980) Appl Surf Sci 5:426
Mei D, Xu DL, Henkelman G (2008) J Catal 258:44
Taylor PA, Rusmussen PB, Ovesen CV, Stoltze P, Chorkendorff I (1992) Surf Sci 261:191
Nerlov J, Chorkendorff I (1999) J Catal 181:271
Taylor PA, Rasmussen PB, Chorkendorff I (1991) J Phys Condens Matter 3:S59
Taylor PA, Rasmussen PB, Chorkendorff I (1995) J Chem Soc Faraday Trans 91:1267
Nakano H, Nakamura I, Fujitani T, Nakamura J (2001) J Phys Chem B 105:1355
Yatsu T, Nishimura H, Fujitani T, Nakamura J (2000) J Catal 191:423
Nakamura I, Nakano H, Fijitani T, Uchijima T, Nakamura J (1999) J Vac Sci Technol A 17:1592
Millar GJ, Rochester CH, Waugh K (1991) J Chem Soc Faraday Trans 87:1491
Millar GJ, Rochester CH, Waugh K (1992) J Chem Soc Faraday Trans 88:1477
Ludviksson A, Zhang R, Campbell CT, Griffiths K (1992) Surf Sci 313:64
Yang Y, Mims CA, Disselkamp RS, Mei D, Kwak Ja-Hun, Szanyi J, Peden CHF, Campbell CT (2008) Catal Lett 125:201
Yang Y, Mims CA, Peden CHF, in preparation
Yang Y, Disselkamp RS, Campbell CT, Szanyi J, Peden CHF, Goodwin JG Jr (2006) Rev Sci Instrum 77 (094104)
Luys M-J, van Oeffelt PH, Pieters P, Ter Veen R (1991) Catal Today 10:283
Luys MJ, van Oeffelt PH, Brouwer WGJ, Pijpers AP, Scholten JJF (1989) Appl Catal 46:161
Hayden BE, Prince K, Woodruff DP, Bradshaw MA (1983) Surf Sci 133:589
Kushida Y, Choi Y, Fujitani T, Uchijima T, Nakamura J (1997) J Surf Sci Soc Jpn 18:478
Madix RJ, Telford SG (1992) Surf Sci 277:246
Nishimura H, Yatsu T, Fujitani T, Uchijima T, Nakamura J (2000) J Mol Cat A Chem 155:3
Meunier FC, Tibiletti D, Goguet A, Reid D, Burch R (2005) Appl Catal A Gen 289:104
Meunier FC, Tibiletti D, Goguet A, Shekhtman S, Hardacre C, Burch R (2007) Catal Today 126:143
Clarke DB, Lee D-K, Sandoval MJ, Bell AT (1994) J Catal 150:81
Acknowledgments
This study was performed at the Institute for Interfacial Catalysis (IIC) at Pacific Northwest National Laboratory (PNNL), and funded by a Laboratory Directed Research and Development (LDRD) grant as part of the Catalysis Initiative program administered by PNNL. The work was carried out in the Environmental Molecular Sciences Laboratory (EMSL) at PNNL, a National Scientific User facility supported by the US Department of Energy Office of Biological and Environmental Research. PNNL is operated by Battelle Memorial Institute for the U.S. Department of Energy. CTC would like to acknowledge the Department of Energy, Office of Basic Energy Sciences, Chemical Sciences Division grant number DE-FG02-96ER14630, for support of this work. CAM gratefully acknowledges PNNL support for his participation in the IIC as a visiting professor. The authors wish to dedicate this paper to the memory of Professor J.M. White.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Y., Mims, C.A., Disselkamp, R.S. et al. Simultaneous MS-IR Studies of Surface Formate Reactivity Under Methanol Synthesis Conditions on Cu/SiO2 . Top Catal 52, 1440–1447 (2009). https://doi.org/10.1007/s11244-009-9320-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-009-9320-3