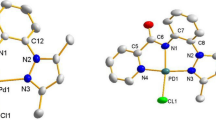
This report describes the catalytic reactivities of a series of Ni and Pd complexes featuring substituted or functionalized indenyl ligands and Ni complexes featuring a PCsp3P type ligand framework. The reactivities of the indenyl complexes encompass styrene homologation (dimerization, trimerization, oligomerization, and polymerization) and hydrosilylation, 1-hexene isomerization, ethylene dimerization and polymerization, phenylacetylene hydrosilylation, coupling reactions of aryl halides with styrene and amines. The reactivities of PCsp3P–Ni complexes is focused on oligomerization of phenylsilane and its addition to styrene, and the addition of aniline to acrylonitrile. The various reactivities discussed herein demonstrate the importance of ligand properties on the catalysis promoted by a given metal.
Similar content being viewed by others
References
S.D. Ittel, L. K. Johnson and M. Brookhart, Chem. Rev. 100 (2000) 1169 and references therein
(a) T.M. Frankom, J.C. Green, A. Nagy, A.K. Kakkar and T.B. Marder, Organometallics 12 (1993) 3688. (b) M.P. Gamasa, J. Gimeno, C. Gonzalez-Bernado, B. M. Martin-Vaca, D. Monti and M. Basseti, Organometallics 15 (1995) 302. (c) J.M. O’Connor and C.P. Casey, Chem. Rev. 87 (1987) 307
For a recent review on the chemistry of group 10 metal indenyl complexes see: D. Zargarian, Coord. Chem. Rev. 233–234 (2002) 157
For recent reviews on pincer complexes see: (a) M. Albrecht and G. van Koten, Angew. Chem. Int. Ed. 40 (2001) 3750. (b) M.E. van der Boom and D. Milstein, Chem. Rev. 103 (2003) 1759.
(a) M. Gozin, A. Weisman, Y. Ben-David and D. Milstein, Nature 364 (1993) 699. (b) M. Gozin, M. Aizenberg, S.-Y. Liou, A. Weisman, Y. Ben-David and D. Milstein, Nature 370 (1994) 42. (c) M.E. van der Boom, S.-Y. Liou, Y. Ben-David, L.J.W. Shimon and D. Milstein, J. Am. Chem. Soc. 120 (1998) 6531. (d) M.E. van der Boom, Y. Ben-David and D. Milstein, J. Am. Chem. Soc. 121 (1999) 6652. (e) R. Cohen, M.E. van der Boom, L.J. W. Shimon, H. Rozenberg and D. Milstein, J. Am. Chem. Soc. 122 (2000) 7723. (f) N. Ashkenazi, A. Vigalok, S. Parthiban, Y. Ben-David, L.J.W. Shimon, J.M. Martin and D. Milstein, J. Am. Chem. Soc. 122 (2000) 8797. (g) M. Kanzelberger, B. Singh, M. Czerw, K. Krogh-Jespersen and A.S. Goldman, J. Am. Chem. Soc. 122 (2000) 11017. (h) D. Morales-Morales, D.W. Lee, Z. Wang and C.M. Jensen, Organometallics 20 (2001) 1144. (i) M. Kanzelberger, X. Zhang, T.J. Emge, A.S. Goldman, J. Zhao, C. Incarvito and J.F. Hartwig, J. Am. Chem. Soc. 125 (2003) 13644
For instance, Ru-PCP compounds catalyze transfer hydrogenation of ketones at high turnover numbers: D. Amoroso, A. Jabri, G.P.A. Yap, D.G. Gusev, E.N. dos Santos and D.E. Fogg, Organometallics 23 (2004) 4047 and references therein
J.T. Singleton (2003) Tetrahedron 59 1837 Occurrence Handle1:CAS:528:DC%2BD3sXhs1ehtLw%3D Occurrence Handle10.1016/S0040-4020(02)01511-9
(a) T.A. Huber, F. Bélanger-Gariépy and D. Zargarian, Organometallics 14 (1995) 4997. (b) M. Bayrakdarian, M.J. Davis, S. Dion, I. Dubuc, F. Bélanger-Gariépy and D. Zargarian, Can. J. Chem. 74 (1996) 2115. (c) T.A. Huber, M. Bayrakdarian, S. Dion, I. Dubuc, F. Bélanger-Gariépy and D. Zargarian, Organometallics 16 (1997) 5811. (d) F.-G. Fontaine, M.-A. Dubois and D. Zargarian, Organometallics 20 (2001) 5156
(a) M.-A. Dubois, R. Wang, D. Zargarian, J. Tian, R. Vollmerhaus, Z. Li and S. Collins, Organometallics 20 (2001) 663. (b) L.F. Groux, D. Zargarian, L.C. Simon and J.B.P. Soares, J. Mol. Catal. A: Chem. 193 (2003) 51
(a) R. Wang, F. Bélanger-Gariépy and D. Zargarian, Organometallics 18 (1999) 5548. (b) R. Wang, L.F. Groux and D. Zargarian, J. Organomet. Chem. 660 (2002) 98. (c) E. Rivera, R. Wang, X.X. Zhu, D. Zargarian and R.Giasson, J. Mol. Catal. A 204–205 (2003) 325.
F.-G. Fontaine, T. Kadkhodazadeh and D. Zargarian, Chem. Commun. (1998) 1253
R. Vollmerhaus F. Bélanger-Gariépy D. Zargarian (1997) Organometallics 16 4762 Occurrence Handle1:CAS:528:DyaK2sXmsVyru70%3D Occurrence Handle10.1021/om970169u
(a) M.-A. Dubois, M.Sc. Thesis, Université de Montréal, 2000. (b) H.-M. Sun, W.-F. Li, X. Han, Q. Shen and Y. Zhang, J. Organomet. Chem. 688 (2003) 132. (c) W.-F. Li, H.-M. Sun, Q. Shen, Y. Zhang and K.-B. Yu, Polyhedron 23 (2004) 1473
(a) F.-G.Fontaine, Ph.D. Thesis, Université de Montréal, 2002. b) F.-G. Fontaine, R.-V. Nguyen and D. Zargarian, Can. J. Chem. 81 (2003) 1299
(a) L.F. Groux, F. Bélanger-Gariépy, D. Zargarian and R. Vollmerhaus, Organometallics 19 (2000) 1507. (b) L.F. Groux and D. Zargarian, Organometallics 20 (2001) 3811. (c) ibid. 22 (2003) 3124. (d) ibid. 4759. (e) D. Gareau, C. Sui-Seng, L.F. Groux, F. Brisse and D. Zargarian, D. Organometallics 24 (2005) 4003
The proposed sequence of electrophilic attacks might also be intramolecular, leading to the formation of cyclic oligomers.
R. Wang L.F. Groux D. Zargarian (2002) Organometallics 21 5531 Occurrence Handle1:CAS:528:DC%2BD38XosVWiu78%3D Occurrence Handle10.1021/om020558a
Analytical data for the hydrosilylation products discussed matched those given in: (a) P.-F. Fu, L. Brard, F.C. Li and T.J. Marks, J. Am. Chem. Soc. 117 (1995) 7157. (b) M. Rubin, T. Schwier and V. Gevorgyan, J. Org.Chem. 67 (2002) 1936
F.-G. Fontaine D. Zargarian (2002) Organometallics 21 401 Occurrence Handle1:CAS:528:DC%2BD3MXptFyisb8%3D Occurrence Handle10.1021/om010757e
Y. Chen C. Sui-Seng S. Boucher D. Zargarian (2005) Organometallics 24 149 Occurrence Handle1:CAS:528:DC%2BD2cXhtVGhs7rP Occurrence Handle10.1021/om0494420
(a) C. Sui-Seng, G.D. Enright and D. Zargarian, Organometallics 23 (2004) 1236. (b) C. Sui-Seng, L.F. Groux and D. Zargarian, Organometallics 25 (2006) 571
R.A. Kelly N.M. Scott S. Diez-Gonzalez E.D. Stevens S.P. Nolan (2005) Organometallics 24 3442 Occurrence Handle1:CAS:528:DC%2BD2MXksVeqsrg%3D Occurrence Handle10.1021/om0501879
For some of the original reports on PCsp3P type pincer complexes see: (a) N.A. Al-Salem, H.D. Empsall, R. Markham, B.L. Shaw and B. Weeks, J. Chem. Soc. Dalton Trans. (1979) 1972. (b) N.A. Al-Salem, W.S. McDonald, R. Markham, M.C. Norton and B.L. Shaw, J. Chem. Soc. Dalton Trans. (1980) 59. (c) C. Crocker, R.J. Errington, R. Markham, C.J. Moulton, K.J. Odell and B.L. Shaw, J. Am. Chem. Soc. 102 (1980) 4373. (d) C. Crocker, R.J. Errington, R. Markham, C.J. Moulton and B.L. Shaw. J. Chem. Soc. Dalton Trans. (1982) 387. (e) C. Crocker, H.D. Empsall, R.J. Errington, E.M. Hyde, W. S. McDonald, R. Markham, M.C. Norton, B.L. Shaw and B. Weeks, J. Chem. Soc. Dalton Trans. (1982) 1217. (f) J.R. Briggs, A.G. Constable, W.S. McDonald and B.L. Shaw, J. Chem. Soc. Dalton Trans. (1982) 1225
(a) A.L. Seligson and W.C. Trogler, Organometallics 12 (1993) 738. (b) A.L. Seligson and W.C. Trogler, Organometallics 12 (1993) 744
S. Sjövall, O.F. Wendt and C. Andersson, J. Chem. Soc. Dalton Trans. (2002) 1396
M. Ohff A. Ohff M.E. Boom Particlevan der D. Milstein (1997) J. Am. Chem. Soc. 119 11687 Occurrence Handle1:CAS:528:DyaK2sXnt1Cjs7s%3D Occurrence Handle10.1021/ja9729692
A. Castonguay, C. Sui-Seng, D. Zargarian and A.L. Beauchamp, Organometallics 25 (2006) 602
(a) H.G. Woo, J.F. Walzer and T.D. Tilley, J. Am. Chem. Soc. 114 (1992) 7047. (b) V.K. Dioumaev, K. Rahimian, F. Gauvin and J.F. Harrod, Organometallics 18 (1999) 2249
(a) L. Fadini and A. Togni, Chem. Commun. (2003) 30. (b) M. Kawatsura and J. Hartwig, Organometallics 20 (2001) 1960. (c) J.M. Seul and S. Park, J. Chem. Soc. Dalton Trans. (2002) 1153
T.E. Müller M. Beller (1998) Chem. Rev. 98 675 Occurrence Handle10.1021/cr960433d
R.A. Benkeser H. Landesman D.J. Foster (1952) J. Am. Chem. Soc. 74 648 Occurrence Handle1:CAS:528:DyaG3sXlvVGksw%3D%3D Occurrence Handle10.1021/ja01123a019
J.F. Hartwig M. Kawatsura S.I. Hauck K.H. Shaughnessy L.M. Alcazar-Roman (1999) J. Org. Chem. 64 5575 Occurrence Handle1:CAS:528:DyaK1MXkt1artL0%3D Occurrence Handle10.1021/jo990408i
J.P. Wolfe S.L. Buchwald (1996) J. Org. Chem. 61 1133 Occurrence Handle1:CAS:528:DyaK28XltlSmsg%3D%3D Occurrence Handle10.1021/jo951844h
N. Kataoka Q. Shelby J.P. Stambuli J.F. Hartwig (2002) J. Org. Chem. 67 5553 Occurrence Handle1:CAS:528:DC%2BD38XltV2mtr8%3D Occurrence Handle10.1021/jo025732j
R. Kuwano M. Utsonomiya J.F. Hartwig (2002) J. Org. Chem. 67 6479 Occurrence Handle1:CAS:528:DC%2BD38XlvFSrtLw%3D Occurrence Handle10.1021/jo0258913
K. Li P.N. Horton M.B. Hursthouse K.K. Hii (2003) J. Organomet. Chem. 665 250 Occurrence Handle1:CAS:528:DC%2BD3sXivVWgtA%3D%3D Occurrence Handle10.1016/S0022-328X(02)02138-1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sui-Seng, C., Castonguay, A., Chen, Y. et al. Catalytic Reactivities of Indenyl–nickel, Indenyl–palladium, and PCsp3P–nickel Complexes. Top Catal 37, 81–90 (2006). https://doi.org/10.1007/s11244-006-0008-7
Issue Date:
DOI: https://doi.org/10.1007/s11244-006-0008-7