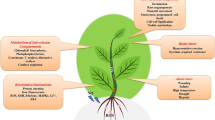
Abstract
Since the era of plant tissue culture bloomed, we have started approaching from a biotechnological perspective to overcome the massive challenges like inducing embryogenesis and organogenesis, initiating rooting, increasing the number of plantlets, establishing a callus from various organs of plants and also enhancing the metabolite content which was a mind-boggling thought once upon a time. Use of various elicitors, altering the media components, the strength of media, pH, precursor feeding etc. have all contributed tremendously in the in-vitro techniques used for culturing rare, endemic and medicinal plants for the commercial purposes. Owing to the demand for the plant products and drugs, the search for the other superior novel methods to increase its quantity and quality has not been stopped. Thus, one such method is the use of chemical compounds with many amino groups which serves as an additional source of nitrogen in the media and these organic compounds are called polyamines. Polyamines are known to play a wide role in plant physiological processes helping them in differentiation, inducing totipotency, increasing cell division and also in molecular signaling. Polyamines have a versatile application in this field ranging from establishing a callus to the elicitation of secondary metabolites. Thus, polyamines can be considered as a boon to the plant tissue culture field. In this review article, we have mainly focused on the in-depth applications of major polyamines like putrescine, spermidine and spermine in the field of plant tissue culture.




Similar content being viewed by others
Abbreviations
- Pas:
-
Polyamines
- Put:
-
Putrescine
- Spd:
-
Spermidine
- Spm:
-
Spermine
- IBA:
-
Indole-3-butyric acid
- NAA:
-
Naphthaleneacetic acid
- BA:
-
Benzylaminopurine
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- DFMA:
-
Difluoromethylarginine
- DFMO:
-
Difluoromethylornithine
- MGBG:
-
Methyl-glyoxyl-bis guanylhydrazone
References
Adkins SW, Samosir YM, Ernawati A, Godwin ID, Drew RA (1998) Control of Ethylene and use of polyamines can optimise the conditions for somatic embryogenesis in coconut (Cocos nucifera L.) and papaya (Carica papaya L.). Acta Hortic 461:459–466
Adkins SW, Samosir YMS, Nikmatullah A, Ogle H (2005) Coconut (Cocus nucifera) in vitro ecology: modifications of headspace and medium additives can optimize somatic embryogenesis. Acta Hortic 692:21–32
Ahmed A, Rahman ME, Tajuddin T, Athar T, Singh M, Garg M, Ahmad S (2014) Effect of nutrient medium, phytohormones and elicitation treatment on in-vitro callus culture of Bacopa monnieri and expression of secondary metabolites. Nat Prod J 4:13–17
Ajithan C, Vasudevan V, Sathish D et al (2019) The influential role of polyamines on the in vitro regeneration of pea (Pisum sativum L.) and genetic fidelity assessment by SCoT and RAPD markers. Plant Cell Tissue Organ Cult 39:245–252
Akhma Z, Yasin MAT, Mahmood M, Shaharuddin NA (2015) Effects of benzyladenine purine and its interaction with polyamines on growth of Spathoglottis plicata PLBs. Turk J Bot 39:245–252
Alcázar R, Planas J, Saxena T et al (2010) Putrescine accumulation confers drought tolerance in transgenic Arabidopsis plants over-expressing the homologous Arginine decarboxylase 2 gene. Plant Physiol Biochem 48:547–552
Altamura MM, Torrigiani P, Capitani F et al (1991) De novo root formation in tobacco thin layers is affected by inhibition of polyamine biosynthesis. J Exp Bot 42:1575–1582
Aragão VPM, Reis RS, Silveira V, Santa-Catarina C (2017) Putrescine promotes changes in the endogenous polyamine levels and proteomic profiles to regulate organogenesis in Cedrela fissilis Vellozo (Meliaceae). Plant Cell Tissue Organ Cult 130:495–505
Arena ME, Pastur GM, Benavides MP, Curvetto N (2005) Polyamines and inhibitors used in successive culture media for in vitro rooting in Berberis buxifolia. N Z J Bot 43:373–380
Arun M, Subramanyam K, Theboral J, Ganapathi A, Manickavasagam M (2014) Optimized shoot regeneration for Indian soybean: the influence of exogenous polyamines. Plant Cell Tissue Organ Cult 117:305–309
Ashok Kumar HG, Ravishankar BV, Murthy HN (2004) The influence of polyamines on androgenesis of Cucumis sativus L. Eur J Hortic Sci 69:201–205
Aydin M, Pour AH, Haliloğlu K, Tosun M (2016) Effect of polyamines on somatic embryogenesis via mature embryo in wheat. Turk J Biol 40:1178–1184
Bais HP, Ravishankar GA (2000) Role of polyamines in the ontogeny of plants and their biotechnological applications. Plant Cell Tissue Organ Cult 69:1–34
Bais HP, George J, Ravishankar GA (1999) Influence of polyamines on growth of hairy root cultures of witloof chicory (Cichorium intybus L. cv. Lucknow Local) and formation of coumarins. J Plant Growth Regul 18:33–37
Bais HP, Madhusudhan R, Bhagyalakshmi N, Rajasekaran T, Ramesh BS, Ravishankar GA (2000) Influence of polyamines on growth and formation of secondary metabolites in hairy root cultures of Beta vulgaris and Tagetes patula. Acta Physiol Plant 22:151–158
Bais H, Sudha G, Ravishankar G (2001) Influence of putrescine, silver nitrate and polyamine inhibitors on the morphogenetic response in untransformed and transformed tissues of Cichorium intybus and their regenerants. Plant Cell Rep 20:547–555
Baron K, Stasolla C (2008) The role of polyamines during in vivo and in vitro development. Vitro Cell Dev Biol Plant 44:384–395
Berberich T, Sagor GH, Tomonobu K (2015) Polyamines in plant stress response. In: Polyamines: a universal molecular nexus for growth, survival, and specialized metabolism, pp 155–168
Besford RT, Richardson CM, Campos JL, Tiburcio AF (1993) Effect of polyamines on stabilization of molecular complexes in thylakoid membranes of osmotically stressed oat leaves. Planta 189:201–206
Burrell M, Hanfrey CC, Murray EJ, Stanley-Wall NR, Michael AJ (2010) Evolution and multiplicity of arginine decarboxylases in polyamine biosynthesis and essential role in Bacillus subtilis biofilm formation. J Biol Chem 285(50):39224–39238
Carman JG, Rodney GR, Fuller J, Ghermay T, Timmis R (2005) Nutrient and hormone levels in Douglas-fir corrosion cavities, mega- gametophytes, and embryos during embryony. Can J For Res 35:2447–3245
Chaturvedi R, Razdan MK, Bhojwani SS (2003) Production of haploids of neem (Azadirachta indica A. Juss.) by anther culture. Plant Cell Rep 21:531–537
Chen LF, Lu W, Sun J, Guo SR, Zhang ZX, Yang YJ (2011) Effects of exogenous spermidine on photosynthesis and carbohydrate accumulation in roots and leaves of cucumber (Cucumis sativus L.) seedlings under salt stress. J Nanjing Agric Univ 34(3):31–36
Cheng L, Zou Y, Ding S et al (2009) Polyamine accumulation in transgenic tomato enhances the tolerance to high temperature stress. J Integr Plant Biol 51:489–499
Chi G, Lin W, Lee JEE, Pua E (1994) Role of polyamines on de novo shoot morphogenesis from cotyledons. Plant Cell Rep 13:323–329
Chiancone B, Tassoni A, Bagni N, Germanà MA (2006) Effect of polyamines on in vitro anther culture of Citrus clementina Hort. ex Tan. Plant Cell Tissue Organ Cult 87:145–153
Chong Perez B, Reyes M, Rojas L, Ocana B, Perez B, Kosky Rafael G, Angenon G (2012) Establishment of embryogenic cell suspension cultures and Agrobacterium-mediated transformation in banana cv. ‘Dwarf Cavendish’ (Musa AAA): effect of spermidine on transformation efficiency. Plant Cell Tissue Organ Cult 111:79–90
Couee I, Hummel I, Sulmon C, Gouesbet G, El Amrani A (2004) Involvement of polyamines in root development. Plant Cell Tissue Organ Cult 76:1–10
De Moura LC, Xavier A, Cláudia A, Batista DS, Miranda NA, Otoni WC (2019) Effect of calcium, BAP and putrescine on somatic embryo induction in juvenile explants of Eucalyptus grandis × E urophylla hybrids. Aust J Crop Sci 13:513–519
De-La-Peña C, Galaz-Ávalos RM, Loyola-Vargas VM (2008) Possible role of light and polyamines in the onset of somatic embryogenesis of Coffea canephora. Mol Biotechnol 39:215–224
Dey A, Gupta K, Gupta B (2014) Role of polyamines in plant–pathogen interactions. In: Anjum NA, Gill SS, Gil R (eds) Plant adaptation to environmental change. CAB International, Wallingford, pp 222–244
Dey A, Hazra AK, Nongdam P, Nandy S, Tikendra L, Mukherjee A, Banerjee S, Mukherjee S, Pandey DK (2019) Enhanced bacoside content in polyamine treated in-vitro raised Bacopa monnieri (L.) Wettst. S Afr J Bot 123:259–269
Diwan R, Malpathak N (2008) Novel technique for scaling up of micropropagated Ruta graveolens shoots using liquid culture systems: a step towards commercialization. New Biotechnol 25(1):85–91
Doctrinal M, Sangwan RS, Sangwan-Norreel BS (1989) In vitro gynogenesis in Beta vulgaris L.: Effects of plant growth regulators, temperature, genotypes and season. Plant Cell Tissue Organ Cult 17:1–12
Ebrahimzadeh H, Shariatpanahi ME, Ahmadi B, Soltanloo H, Lotfi M, Zarifi E (2018) Efficient parthenogenesis induction and in vitro haploid plant regeneration in cucumber (Cucumis sativus L.) using putrescine, spermidine, and cycocel. J Plant Growth Regul 37:1127–1134
El-dawayati MM, Ghazzawy HS, Munir M (2018) Somatic embryogenesis enhancement of date palm cultivar Sewi using different types of polyamines and glutamine amino acid concentration under in-vitro solid and liquid media conditions. Int J Biosci 12:149–159
Emilio M, De G, Ida J, Eddo R (2007) Efficient method of micropropagation and in vitro rooting of teak (Tectona grandis L.) focusing on large-scale industrial plantations. Ann For Sci 64:73–78
Evans PT, Malmberg RL (1989) Do Polyamines have roles in plant development? Annu Rev Plant Physiol Plant Mol Biol 40:235–269
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–221
Fazilati M, Forghani AH (2015) The role of polyamine to increasing growth of plant: As a key factor in health crisis. Int J Health Syst Disaster Manag 3:89–94
Flores HE, Galston AW (1982) Polyamines and plant stress: Activation of putrescine biosynthesis by osmotic shock. Science 217:1259–1261
Fraguas CB, Villa F, Lima GPP (2009) Evaluation of exogenous application of polyamines on callus growth of Mangaba tree (Hancornia speciosa Gomes). Rev Bras Frutic 31:1206–1210
Ganesan M, Narayanasamy J (2006) Influence of cytokinis, auxins and polyamines on in vitro mass multiplication of cotton (Gossypium hirsutum L.cv.SVPR2). Indian J Exp Biol 44:506–513
Ghosh B (2000) Polyamines and plant alkaloids. Indian J Exp Biol 38:1086–1091
Gonzalez ME, Marco F, Minguet EG et al (2011) Perturbation of spermine synthase gene expression and transcript profiling provide new insights on the role of the tetraamine spermine in Arabidopsis defense against pseudomonas viridiflava. Plant Physiol 156:2266–2277
Gow WP, Chen JT, Chang WC (2008) Influence of growth regulators on direct embryo formation from leaf explants of Phalaenopsis orchids. Acta Physiol Plant 30:507
Hao G, Ji H, Li Y, Shi R, Wang J, Feng L, Huang L (2012) Exogenous ABA and polyamines enhanced salvianolic acids contents in hairy root cultures of Salvia miltiorrhiza Bge.f.alba. Plant Omics J 5:446–452
Hausman JF, Gevers C, Gaspar T (1994) Involvement of putrescine in the inductive rooting phase of poplar shoots raised in vitro. Physiol Plant 92:201–206
He L, Nada K, Tachibana S (2002) Effects of spermidine pretreatment through the roots on growth and photosynthesis of chilled cucumber plants (Cucumis sativus L.). Chem Pharm Bull 71:490–498
Hema BP, Murthy HN (2008) Improvement of in vitro androgenesis in niger using amino acids and polyamines. Biol Plant 52:121–125
Hwang SJ, Kim KS, Pyo BS, Hwang B (1999) Saponin production by hairy root cultures of Panax ginseng CA meyer: influence of PGR and polyamines. Biotechnol Bioprocess Eng 4:309–312
Ioannidis NE, Kotzabasis K (2007) Effects of polyamines on the functionality of photosynthetic membrane in vivo and in vitro. Biochim Biophys Acta 1767:1372–1382
Jarvis BC, Shannon PRM, Yasmin S (1983) Involvement of polyamines with adventitious root development in stem cuttings of mung bean. Plant Cell Physiol 24:677–683
Jiménez-Bremont JF, Marina M, Guerrero-González ML, Rossi FR, Sánchez-Rangel D, Rodríguez-Kessler M, Ruiz OA, Gárriz A (2014) Physiological and molecular implications of plant polyamine metabolism during biotic interactions. Front Plant Sci 5:95
Joshee N, Harris D, Yadav A, Yadav AK (2007) Influence of explant selection and culture conditions on organogenesis and germplasm conservation in Bacopa monnieri (L.) Wettst. Acta Hortic 756:119–128
Kabir A, Suresh Kumar G (2013) Binding of the biogenic polyamines to deoxyribonucleic acids of varying base composition: base specificity and the associated energetics of the interaction. PLoS ONE
Kakkar RK, Nagar PK, Ahuja PS, Rai VK (2000) Polyamines and plant morphogenesis. Biol Plant 43:1–11
Kaur-Sawhney R, Tiburcio AF, Galston AW (1988) Spermidine and flower-bud differentiation in thin-layer explants of tobacco. Planta 173:282–284
Kaur-Sawhney R, Tiburcio AF, Altabella T, Galston AW (2003) Polyamines in plants: an overview. J Cell Mol Biol 2:1–12
Kaur-sawhney R, Kandpal G, Mcgonigle B, Galston AW (1990) Further experiments on spermidine-mediated floral-bud formation in thin-layer explants of Wisconsin 38 tobacco. Planta 181:212–215
Kevers C, Le Gal N, Monteiro M et al (2000) Somatic embryogenesis of Panax ginseng in liquid cultures: a role for polyamines and their metabolic pathways. Plant Growth Regul 31:209–214
Kevers C, Gaspar T, Dommes J (2002) The beneficial role of different auxins and polyamines at successive stages of somatic embryo formation and development of Panax ginseng in vitro. Plant Cell Tissue Organ Cult 70:181–188
Khalil SA, Kamal N, Sajid M, Ahmad Nisar Zamir R, Ahmad Naveed Ali S (2016) Synergism of polyamines and plant growth regulators enhanced morphogenesis, steviosides content, and production of commercially important natural antioxidants in Stevia rebaudiana Bert. Vitro Cell Dev Biol Plant 52:174–184
Khosh-Khui M, Sink KC (1982) Rooting-enhancement of Rosa hybrid for tissue culture propagation. Sci Hortic 17:371–376
Kim TE, Kim SK, Han TJ et al (2002) ABA and polyamines act independently in primary leaves of cold-stressed tomato (Lycopersicon esculentum). Physiol Plant 115:370–376
Kim JK, Baskar TB, Park SU (2016) Silver nitrate and putrescine enhance in vitro shoot organogenesis in Polygonum tinctorium. Biosci Biotechnol Res Asia 13:53–58
Krishnamurthy R (1991) Amelioration of salinity effect in salt tolerant rice (oryza sativa l.) by foliar application of putrescine. Plant Cell Physiol 32:699–703
Kumar V, Sharma EA, Chayapathy EB, Prasad N, Bhaskar H, Parvatam GE, Gokare GE, Ravishankar GA (2007) Direct shoot bud induction and plant regeneration in Capsicum frutescents Mill.: influence of polyamines and polarity. Acta Physiol Plant 29:11–18
Kumar V, Giridhar P, Chandrashekar A, Ravishankar GA (2008) Polyamines influence morphogenesis and caffeine biosynthesis in in vitro cultures of Coffea canephora P. ex Fr. Acta Physiol Plant 30:217–223
Kusano T, Berberich T, Tateda C, Takahashi Y (2008) Polyamines: essential factors for growth and survival. Planta 228:367–381
Laukkanen H, Sarjala T (1997) Effect of exogenous polyamines on scots pine callus in vitro. J Plant Physiol 150:167–172
Lee SY, Baskar TB, Kim JK, Park SU (2016) Enhanced shoot organogenesis in Aloe saponaria following treatment with ethylene inhibitors and polyamines. Biosci Biotechnol Res Asia 13:17–21
Liu Y, Liang H, Lv X et al (2016) Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiol Biochem 100:113–129
Malabadi Ravindra B, Nataraja K (2007) Putrescine influences somatic embryogenesis and plant regeneration in Pinus gerardiana Wall. Am J Plant Physiol 2:107–114
Martı G, Curvetto EEÆN (2007) Role of polyamines during in vitro rhizogenesis of Nothofagus nervosa using successive culture media. New For 34:83–93
Martin KP (2003) Plant regeneration through somatic embryogenesis on Holostemmaada-kodien, a rare medicinal plant. Plant Cell Tissue Organ Cult 72:79–82
Martin-Tanguy J, Carre M (1993) Polyamines in grapevine microcuttings cultivated in vitro. Effects of amines and inhibitors of polyamine biosynthesis on polyamine levels and microcutting growth and development. Plant Growth Regul 13:269–280
Martnez LE, Aguero CB, Lopez ME, Galmarini CR (2000) Improvement of in vitro gynogenesis induction in onion (Allium cepa L.) using polyamines. Plant Sci 156:221–226
Mattoo AK, Fatima T, Upadhyay RK, Handa AK (2015) Polyamines in plants: biosynthesis from arginine, and metabolic, physiological and stress-response roles. In: D’Mello JPF (ed) Amino acids in higher plants. CAB International, Wallingford, pp 177–194
Minocha SC, Minocha R (1995) Role of polyamines in somatic embryogenesis. In: Bajaj YPS (ed) Somatic embryogenesis and synthetic seed I, biotechnology in agriculture and forestry. Springer, Berlin, pp 53–70
Mustafavi SH, Naghdi Badi H, Sekara A et al (2018) Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiol Plant 40:102
Nakagawa R, Ogita S, Kubo T, Funada R (2006) Effect of polyamines and L-ornithine on the development of proembryogenic masses of Cryptomeria japonica. Plant Cell Tissue Organ Cult 85:229–234
Niemi K, Sarjala T, Chen X, Häggman H (2002) Spermidine and methylglyoxal bis(guanylhydrazone) affect maturation and endogenous polyamine content of Scots pine embryogenic cultures. J Plant Physiol 159:1155–1158
Niemi K, Sarjala T, Chen X, Häggman H (2007) Spermidine and the ectomycorrhizal fungus Pisolithus tinctorius synergistically induce maturation of Scots pine embryogenic cultures. J Plant Physiol 164:629–635
Nikam TD, Ghorpade RP, Nitnaware KM, Ahire ML, Lokhande VH, Chopra A (2013) Micropropagation and non-steroidal anti-inflammatory and anti-arthritic agent boswellic acid production in callus cultures of Boswellia serrata Roxb. Physiol Mol Biol Plants 19:105–116
Palavan-ünsal N (1987) Polyamine metabolism in the roots of Phaseolus vulgaris. Interaction of the inhibitors of polyamine biosynthesis with putrescine in growth and polyamine biosynthesis. Plant Cell Physiol 28:565–572
Parale A, Barmukh R, Nikam T (2010) Influence of organic supplements on production of shoot and callus biomass and accumulation of bacoside in Bacopa monnieri (L.) Pennell. Physiol Mol Biol Plants 16:167–175
Park E, Bae H, Park WT, Kim YB, Chae SC, Park SU (2012) Improved shoot organogenesis of gloxinia (Sinningia speciosa) using silver nitrate and putrescine treatment. Plant OMICS 5:6–9
Parvin S, Lee OR, Sathiyaraj G, Khorolragchaa A, Kim YJ, Yang DC (2014) Spermidine alleviates the growth of saline-stressed ginseng seedlings through antioxidative defense system. Genetics 53:70–78
Paul A, Mitter K, Raychaudhuri SS (2009) Effect of polyamines on in vitro somatic embryogenesis in Momordica charantia L. Plant Cell Tissue Organ Cult 97:303–311
Ponce MT, Guinazu M, Tizio R (2002a) Effect of putrescine on embryo development in the stenospermocarpic grape cvs Emperatriz and Fantasy. Vitis 41:53–54
Ponce MT, Guinazu M, Tizio R (2002b) Improved in vitro embryo development of stenospermic grape by putrescine. Biocell 26:263–266
Pottosin I, Shabala S (2014) Polyamines control of cation transport across plant membranes: implications for ion homeostasis and abiotic stress signaling. Front Plant Sci 5:1–16
Protacio C, Flores H (1992) The role of polyamines in potato tuber formation. In Vitro Cell Dev Biol 28:81–86
Rajesh MK, Karun A (2014) Polyamine-induced somatic embryogenesis and plantlet regeneration in vitro from plumular explants of dwarf cultivars of coconut (Cocos nucifera). Indian J Agric Sci 84:527–530
Rajesh MK, Radha E, Karun A, Parthasarathy VA (2003) Plant regeneration from embryo-derived callus of oil palm—the effect of exogenous polyamines. Plant Cell Tissue Organ Cult 75:41–47
Redha A, Suleman P (2011) Effects of exogenous application of polyamines on wheat anther cultures. Plant Cell Tissue Organ Cult 105:345–353
Reis RS, de Vale EM, Heringer AS, Santa-Catarina C, Silveira V (2016) Putrescine induces somatic embryo development and proteomic changes in embryogenic callus of sugarcane. J Proteom 130:170–179
Rey M, Diaz Sala C, Roberto R (1994) Exogenous polyamines improve rooting of hazel microshoots. Plant Cell Tissue Organ Cult 36:303–308
Rezvanypour S, Hatamzadeh A, Elahinia SA, Asghari HR (2015) Exogenous polyamines improve mycorrhizal development and growth and flowering of Freesia hybrida. J Hortic Res 23(2):17–25
Rodríguez AA, Maiale SJ, Menéndez AB, Ruiz OA (2009) Polyamine oxidase activity contributes to sustain maize leaf elongation under saline stress. J Exp Bot 60:4249–4262
Rodríguez-Kessler M, Ruiz OA, Maiale S et al (2008) Polyamine metabolism in maize tumors induced by Ustilago maydis. Plant Physiol Biochem 46:805–814
Roy M, Wu R (2001) Arginine decarboxylase transgene expression and analysis of environmental stress tolerance in transgenic rice. Plant Sci 160:869–875
Rubio-rodríguez E, Trejo-tapia G, López-laredo AR, Medina-pérez V, Trejo-espino JL (2019) Influence of spermine and nitrogen deficiency on growth and secondary metabolites accumulation in Castilleja tenuiflora Benth. cultured in a RITA ® temporary immersion system. Eng Life Sci 19:944–954
Sabapathy S, Nair H (1992) Invitro propagation of taro, with spermine, arginine, and ornithine. Plant Cell Rep 11:290–294
Sagor GHM, Berberich T, Takahashi Y et al (2013) The polyamine spermine protects Arabidopsis from heat stress-induced damage by increasing expression of heat shock-related genes. Transgenic Res 22:595–605
Saiprasad GVS, Raghuveer P, Khetarpal S, Chandra R (2004) Effect of various polyamines on production of protocorm-like bodies in orchid—Dendrobium ‘Sonia.’ Sci Hortic 100:161–168
Sakhanokho HF, Ozias-Akins P, Lloyd MO, Chee PW (2005) Putrescine enhances somatic embryogenesis and plant regeneration in upland cotton. Plant Cell Tissue Organ Cult 81:91–95
Sathish D, Vasudevan V, Theboral J, Elayaraja D (2018) Efficient direct plant regeneration from immature leaf roll explants of sugarcane (Saccharum officinarum L.) using polyamines and assessment of genetic fidelity by SCoT markers. In Vitro Cell Dev Biol Plant 54:399–412
Sathish D, Theboral J, Vasudevan V, Pavan G, Ajithan C, Appunu C, Manickavasagam M (2020) Exogenous polyamines enhance somatic embryogenesis and Agrobacterium tumefaciens-mediated transformation efficiency in sugarcane (Saccharum spp. hybrid). Vitro Cell Dev Biol Plant 56:29–40
Satish L, Rency AS, Rathinapriya P, Ceasar SA, Pandian S, Rameshkumar R, Rao TB, Balachandran SM, Ramesh M (2016) Influence of plant growth regulators and spermidine on somatic embryogenesis and plant regeneration in four Indian genotypes of finger millet (Eleusine coracana (L.) Gaertn). Plant Cell Tissue Organ Cult 124:15–31
Scholten HJ (1998) Effect of polyamines on the growth and development of some horticultural crops in micropropagation. Sci Hortic 77:83–88
Serafini-Fracasini D, Di Sandro A, Del Duca S (2010) Spermine delays leaf senescence in Lactuca sativa and prevents the decay of chloroplast photosystems. Plant Physiol Biochem 48:602–611
Setia N, Setia RC (2018) Polyamines: an overview and prospects in crop improvement. Crop Improv Strateg App 376–393
Shalaby TA (2007) Factors affecting haploid induction through in vitro gynogenesis in summer squash (Cucurbita pepo L.). Sci Hortic 115:1–6
Sharma M, Gupta R, Khajuria RK, Mallubhotla S, Ahuja A (2015) Bacoside biosynthesis during in vitro shoot multiplication in Bacopa monnieri (L.) Wettst. grown in Growtek and air lift bioreactor. Indian J Biotechnol 14:547–551
Shen HJ, Galston AW (1985) Correlations between polyamine ratios and growth patterns in seedling roots. Plant Growth Regul 3:353–363
Shetty K, Korus Roger A, Crawford DL (1989) Growth kinetics and phenolics production in Glycine Max cell suspension cultures: effect of microbial elicitor, calcium, polyamines, and organic osmolytes. Appl Biochem Biotechnol 20–21:825–843
Silveira V, Santa-Catarina C, Tun NN, Scherer GFE, Handro W, Guerra MP, Floh EIS (2006) Polyamine effects on the endogenous polyamine contents, nitric oxide release, growth and differentiation of embryogenic suspension cultures of Araucaria angustifolia (Bert.) O. Ktze. Plant Sci 171:91–98
Sivanandhan G, Salammal T (2011) The effect of polyamines on the efficiency of multiplication and rooting of Withania somnifera (L.) Dunal and content of some withanolides in obtained plants. Acta Physiol Plant 33:2279–2288
Srivastava MNS (2017) Elicitation: a stimulation of stress in in vitro plant cell / tissue cultures for enhancement of secondary metabolite production. Phytochem Rev 16:1227–1252
Steiner N, Santa-Catarina C, Silveira V, Floh EIS, Guerra MP (2007) Polyamine effects on growth and endogenous hormones levels in Araucaria angustifolia embryogenic cultures. Plant Cell Tissue Organ Cult 89:55–62
Sudha G, Ravishankar GA (2003) Putrescine facilitated enhancement of capsaicin production in cell suspension cultures of Capsicum frutescens. J Plant Physiol 160:339–346
Sundararajan S, Sivakumar HP, Nayeem S et al (2020) Influence of exogenous polyamines on somatic embryogenesis and regeneration of fresh and long-term cultures of three elite indica rice cultivars. Cereal Res Commun
Sunderland N (1974) Anther culture: a progress report. Sci Prog 59:527–549
Tabart J, Franck T, Kevers C, Dommes J (2015) Effect of polyamines and polyamine precursors on hyperhydricity in micropropagated apple shoots. Plant Cell Tissue Organ Cult 120:11–18
Takahashi T, Kakehi J (2010) Polyamines: ubiquitous polycations with unique roles in growth and stress responses. Ann Bot 105:1–6
Takeda T, Hayakawa F, Oe K, Matsuoka H (2002) Effects of exogenous polyamines on embryogenic carrot cells. Biochem Eng J 12:21–28
Tang W, Newton RJ (2005) Polyamines promote root elongation and growth by increasing root cell division in regenerated Virginia pine (Pinus virginiana Mill.) plantlets. Plant Cell Rep 24:581–589
Tang W, Newton RJ, Outhavong V (2004) Exogenously added polyamines recover browning tissues into normal callus cultures and improve plant regeneration in pine. Physiol Plant 122:386–395
Tarenghi E, Carr M (1995) Effects of inhibitors of polyamine biosynthesis and of polyamines on strawberry microcutting growth and development. Plant Cell Tissue Organ Cult 42:47–55
Thiruvengadam M (2012) Influence of polyamines on in vitro organogenesis in bitter melon (Momordica charantia L.). J Med Plants Res 6:3579–3585
Thiruvengadam M, Praveen N, Kim EH, Chung IM, Rekha K, Jayabalan N (2013) Effect of exogenous polyamines enhances somatic embryogenesis via suspension cultures of spine gourd (Momordica dioica Roxb. ex. Willd.). Aust J Crop Sci 7:446–453
Todorova D, Katerova Z, Sergiev I, Alexieva V (2014) Polyamines - involvement in plant stress tolerance and adaptation. CABI, Wallingford, pp 194–221
Torrigiani P, Altamura MM, Capitani F et al (1989) De novo root formation in thin cell layers of tobacco: changes in free and bound polyamines. Physiol Plant 77:294–301
Vasudevan A, Selvaraj N, Ganapathi A (2008) Leucine and spermidine enhance shoot differentiation in cucumber (Cucumis sativus L.). Vitro Cell Dev Biol Plant 44:300–306
Vasudevan V, Subramanyam K, Elayaraja D (2017) Assessment of the efficacy of amino acids and polyamines on regeneration of watermelon (Citrullus lanatus Thunb.) and analysis of genetic fidelity of regenerated plants by SCoT and RAPD markers. Plant Cell Tissue Organ Cult 8:1–7
Venkatachalam L, Bhagyalakshmi N (2008) Spermine-induced morphogenesis and effect of partial immersion system on the shoot cultures of Banana. Appl Biochem Biotechnol 151:502–511
Viu AFM, Viu MA, Tavares AR, Vianello F, Lima GPP (2009a) Endogenous and exogenous polyamines in the organogenesis in Curcuma longa L. Sci Hortic 121:501–504
Viu AFM, Viu MAO, Tavares AR et al (2009b) Endogenous and exogenous polyamines in the organogenesis in Curcuma longa L. Sci Hortic 121:501–504
Walters DR (2003) Polyamines and plant disease. Phytochemistry 64:97–107
Wang G, Xu Z, Chia TF, Chua NH (1993) In Vitro flowering of orchid (Dendrobium Candidum). Current Plant Sci Biotechnol Agric 15:373–378
Wolff EC, Park MH (2015) Role of the polyamine spermidine as a pre-cursor for hypusine modification in eIF5A. In: Kusano T, Suzuki H (eds) Polyamines. Springer, Tokyo, pp 121–130
Yoshioka T, Yamagata H, Jthoh A, Deno H, Fujita Y (1989) Effects of exogenous polyamines on tropane alkaloid production by a root culture of Duboisia myoporoides. Planta Med 55:523–524
Zhu C, Chen Z (2005) Role of polyamines in adventitious shoot morphogenesis from cotyledons of cucumber in vitro. Plant Cell Tissue Organ Cult 81:45–53
Zimmerman JL (1993) Somatic embryogenesis: a model for early development in higher plants. Plant Cell 5:1411–1423
Acknowledgements
We would like to acknowledge Miss. Smita Dhantal, PhD Scholar, Dept. of English and Cultural Studies, CHRIST (Deemed To Be University) for proof reading the article and commenting on the grammatical and linguistic aspects.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Sergio J. Ochatt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rakesh, B., Sudheer, W.N. & Nagella, P. Role of polyamines in plant tissue culture: An overview. Plant Cell Tiss Organ Cult 145, 487–506 (2021). https://doi.org/10.1007/s11240-021-02029-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02029-y