Abstract
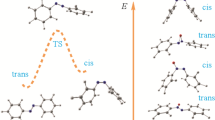
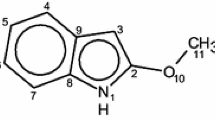
The optimized structures of all isomers of HBI, HBO, HBT, HPyBI, HPyBO, and HPyBT compounds were obtained using the potential energy surface method at the B3LYP/6-311++G(d,p) level of theory. Four isomers and three transition states of their transformations for each compound of HBO, HBT, HPyBO, and HPyBT and two isomers and one transition state for each HBI and HPyBI compounds were found. Energetics, thermodynamic properties, rate constants, and equilibrium constants of their transformations were determined.







Similar content being viewed by others
References
Lim S-J, Seo J, Park SY (2006) J Am Chem Soc 128:14542–14547. doi:10.1021/ja0637604
Ma DG, Liang FS, Wang LX, Lee ST, Hung LS (2002) Chem Phys Lett 358:24–28. doi:10.1016/S0009-2614(02)00546-8
Paterson MJ, Robb MA, Blancafort L, DeBellis AD (2005) J Phys Chem A 109:7527–7537. doi:10.1021/jp051108+
Klymchenko AS, Pivovarenko VG, Demchenko AP (2003) J Phys Chem A 107:4211–4216. doi:10.1021/jp027315g
Sarkar M, Ray JG, Sengupta PK (1996) Spectrochim Acta A Mol Biomol Spectrosc 52:275–278. doi:10.1016/0584-8539(95)01622-8
Klymchenko AS, Demchenko AP (2002) Langmuir 18:5637–5639. doi:10.1021/la025760x
Das K, Sarkar N, Majumdar D, Bhattacharyya K (1992) Chem Phys Lett 198:443–448. doi:10.1016/0009-2614(92)80025-7
Das K, Sarkar N, Ghosh AK, Majumdar D, Nath DN, Bhattacharyya K (1994) J Phys Chem 98:9126–9132. doi:10.1021/j100088a006
Mosquera M, Penedo JC, Rios Rodriguez MC, Rodriguez-Preito F (1996) J Phys Chem 100:5398–5407. doi:10.1021/jp9533638
Rios MA, Rios MC (1998) J Phys Chem 102:1560–1567
Rios MA, Rios MC (1995) J Phys Chem 99:12456–12460. doi:10.1021/j100033a014
Ohshima A, Momotake A, Nagahata R, Arai T (2005) J Phys Chem A 109:9731–9736. doi:10.1021/jp053702p
Fernandez-Ramos A, Rodriguez-Otero J, Rios MA, Soto J (1999) J Mol Struct THEOCHEM 489:255–262. doi:10.1016/S0166-1280(99)00062-7
Lochbrunner S, Stock K, Riedle E (2004) J Mol Struct 700:13–18. doi:10.1016/j.molstruc.2004.01.038
Abou-Zied OK, Jimenze R, Romesberg FE (2001) J Am Chem Soc 123:4613–4614. doi:10.1021/ja003647s
Dupradeau FY, Case DA, Yu C, Jimenez R, Romesberg FE (2005) J Am Chem Soc 127:15612–15617. doi:10.1021/ja054607x
Wang H, Zhang H, Abou-Zied OK, Yu C, Romesberg FE, Glasbeek M (2003) Chem Phys Lett 367:599–608. doi:10.1016/S0009-2614(02)01741-4
Chou P-T, Studer SL, Martinez ML (1991) Chem Phys Lett 178:393–398. doi:10.1016/0009-2614(91)90271-A
Chou P-T, Martinez ML, Studer SL (1992) Chem Phys Lett 195:586–590. doi:10.1016/0009-2614(92)85567-T
Lochbrunner S, Wurzer AJ, Riedle E (2003) J Phys Chem A 107:10580–10590. doi:10.1021/jp035203z
Vivie-Riedle R, De Waele V, Kurtz L, Riedle E (2003) J Phys Chem A 107:10591–10599. doi:10.1021/jp035204r
Rodriguez-Preito F, Rios Rodriguez MC, Mosquera Gonzalez M, Rios Fernandez MA (1994) J Phys Chem 98:8666–8672. doi:10.1021/j100086a014
Vazquez SR, Rodriguez MCR, Mosquera M, Rodriguez-Preito F (2007) J Phys Chem A 111:1814–1826. doi:10.1021/jp0653813
Becke AD (1993) J Chem Phys 98:5648–5652. doi:10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2006) Gaussian 03, Revision C.02. Gaussian Inc., Wallingford, CT
Sabin JR, Trickey SB, Apell SP, Oddershede J (2000) Int J Quantum Chem 77:358. doi:10.1002/(SICI)1097-461X(2000)77:1<358::AID-QUA35>3.0.CO;2-D
Koopmans T (1933) Physica 1:104–113. doi:10.1016/S0031-8914(34)90011-2
Wanno B, Ruangpornvisuti V (2005) Chem Phys Lett 415:176–182. doi:10.1016/j.cplett.2005.08.141
Wanno B, Ruangpornvisuti V (2006) J Mol Struct 787:76–89. doi:10.1016/j.molstruc.2005.11.006
Wanno B, Ruangpornvisuti V (2006) J Mol Struct THEOCHEM 766:159–164. doi:10.1016/j.theochem.2006.04.011
Wanno B, Ruangpornvisuti V (2006) J Mol Struct THEOCHEM 775:113–120. doi:10.1016/j.theochem.2006.08.017
Navakhun K, Ruangpornvisuti V (2006) J Mol Struct THEOCHEM 772:23–30. doi:10.1016/j.theochem.2006.06.013
Navakhun K, Ruangpornvisuti V (2007) J Mol Struct THEOCHEM 806:145–153. doi:10.1016/j.theochem.2006.11.016
Keawwangchai S, Tuntulani T, Ruangpornvisuti V (2007) J Mol Struct 832:16–25. doi:10.1016/j.molstruc.2006.07.039
Ruangpornvisuti V (2007) Struct Chem 18:977–984. doi:10.1007/s11224-007-9258-7
Thipyapong K, Ruangpornvisuti V (2008) J Mol Struct 891:1–10. doi:10.1016/j.molstruc.2008.02.004
Wigner EZ (1932) Phys Chem B 19:203–204
Hirschfelder JO, Wigner E (1939) J Chem Phys 7:616–628. doi:10.1063/1.1750500
Bell RP (1980) The tunnel effect in chemistry. Chapman and Hall, London
Leffler JE (1953) Science 117:340–341. doi:10.1126/science.117.3039.340
Hammond GS (1955) J Am Chem Soc 77:334–338. doi:10.1021/ja01607a027
Pearson RG (2001) Hard and soft acids and bases. Dowden (Hutchison & Ross), Stroudsburg, PA
Acknowledgments
This work was partially supported from financial support via the Thailand Research Fund (TRF), Grant No. MRG5080266 and the Center of Excellent for Innovation in Chemistry (PERCH-CIC), Commission on Higher Education, Ministry of Education. This work was partially supported by the National Center of Excellence for Petroleum, Petrochemicals and Advanced Materials.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suwattanamala, A., Ruangpornvisuti, V. Isomeric structures of benzimidazole, benzoxazole, and benzothiazole derivatives, their electronic properties and transformations. Struct Chem 20, 619–631 (2009). https://doi.org/10.1007/s11224-009-9454-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-009-9454-8