Abstract
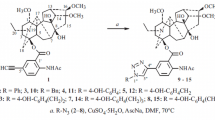
Lappaconitine and N-deacetyllappaconitine derivatives containing bromine and iodine atoms in the aromatic moiety were synthesized. The Heck cross-coupling of these halides with ethyl acrylate or 2-methyl-5-vinylpyridine afforded new olefinated lappaconitine derivatives.
Similar content being viewed by others
References
N. V. Anferova, I. Yu. Bagryanskaya, Yu. V. Gatilov, S. A. Osadchii, M. M. Shakirov, E. E. Shults, and G. A. Tolstikov, Izv. Akad. Nauk, Ser. Khim., 2003, 2363 [Russ. Chem. Bull., Int. Ed., 2003, 52, 2500].
M. D. Mashkovskii, Lekarstvennye sredstva [Drugs], Torsing, Kharkov, 1998, 1, 380 (in Russian).
R. D. Kurbanov and T. A. Abdullaev, Klinicheskaya Meditsina [Clinical Medicine], 1988, 66, No. 10, 52 (in Russian).
A. S. Smetnev, S. P. Golitsin, and E. Z. Levin, Terapevticheskii Arkhiv [Therapeutical Archive], 1988, 60, No. 8, 34 (in Russian).
V. S. Gasilin, E. V. Dorofeev, and N. I. Rozova, Kardiologiya [Cardiology], 1990, 30, No. 9, 30 (in Russian).
M. S. Yunusov, Bashkir. Khim. Zh. [Bashkir. Chem. J.], 1997, 4, No. 4, 16 (in Russian).
F. N. Dzhakhangirov, M. N. Sultankhadzhaev, B. Tashkhodzhaev, and B. T. Salimov, Khim. Prirod. Soedin., 1997, 254 [Chem. Nat. Compd., 1997 (Engl. Transl.)].
H. K. Desai, B. P. Hart, R. W. Caldwell, J. Huang, and S. W. Pelletier, J. Nat. Prod., 1998, 61, 743.
F. N. Dzhakhangirov, Diterpenovye alkaloidy — novyi klass prirodnykh veshchestv s antiaritmicheskoi aktivnost’yu [Diterpene Alkaloids — A New Class of Natural Compounds with Antiarrhythmic Activity], in Azotistye geterotsikly I alkaloidy [Nitrogen Heterocycles and Alkaloids], Eds V. G. Kartsev and G. A. Tolstikov, Iridium-press, 2001, U2, 296 (in Russian).
J. F. Heubach and A. Schuele, Planta Med., 1998, 64, 22.
T. G. Tolstikova, T. V. Voevoda, and M. P. Dolgikh, Eksp. Klinich. Farmak. [Exp. Clinic. Pharmacol.], 2001, 64, No. 4, 7 (in Russian).
U. T. Gutser, J. Friese, J. F. Heubach, T. Matthiesen, N. Selve, B. Wilffert, and J. Gleitz, Arch. Pharm., 1998, 357, 39.
Pat. RF 2180583; Byul. izobret. [Invention Bull.], 2002, 148 (in Russian).
S. A. Osadchii, N. A. Pankrushina, M. M. Shakirov, E. E. Shults, and G. A. Tolstikov, Izv. Akad. Nauk, Ser. Khim., 2000, 552 [Russ. Chem. Bull., Int. Ed., 2000, 49, 557].
N. A. Pankrushina, I. A. Nikitina, N. V. Anferova, S. A. Osadchii, M. M. Shakirov, E. E. Shults, and G. A. Tolstikov, Izv. Akad. Nauk, Ser. Khim., 2003, 2354 [Russ. Chem. Bull., Int. Ed., 2003, 52, 2490].
S. A. Ross and S. W. Pelletier, Heterocycles, 1991, 32, 1307.
N. V. Malykhina, S. A. Osadchii, M. M. Shakirov, E. E. Shults, and G. A. Tolstikov, Dokl. Akad. Nauk, 2004, 394, 343 [Dokl. Chem., 2004 (Engl. Transl.)].
Q.-H. Chen and F.-P. Wang, Chines Chem. Lett., 2001, 12, 421.
R. F. Heck, Palladium Reagents in Organic Synthesis, Academic Press, New York, 1985, 276.
J. Tsuji, Palladium Reagents and Catalysts: Innovation in Organic Chemistry, Wiley, Chichester, 1995, 95.
M. Larhed and A. Hallberg, The Heck Reaction, in Handbook of Organopalladium Chemistry for Organic Synthesis, Wiley, New York, 2002, 1133.
I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 3009.
US Pat. 5068392; Chem. Abstrs, 1992, 116, 83386.
P. A. Petyunin and M. E. Konshin, Zh. Obshch. Khim., 1957, 27, 475 [J. Gen. Chem. USSR, 1957, 27 (Engl. Transl.)].
P. Beinker, J. R. Hanson, N. Meindl, and I. C. R. Medina, J. Chem. Res. S., 1998, 4, 204.
P. A. Galenko-Yaroshevskii, O. A. Chekanova, V. V. Skibitskii, V. V. Bartashevich, A. I. Khankoeva, and T. I. Polyashova, Byul. Eksp. Biol. Med., Farmak. Toksik. [Bull. Exp. Biol. Med., Pharmac. Toxic. (Engl. Transl.)].
D. Barker, M. D. McLeod, M. A. Brimble, and G. P. Savage, Tetrahedron Lett., 2001, 42, 1785.
H. Schulze, Arch. Pharm. Ber. Deutsch. Pharm. Ges., 1922, 260, 230.
G. B. Shul’pin, Organicheskie reaktsii, kataliziruemye kompleksami metallov [Metal Complex Catalyzed Organic Reactions], Nauka, Moscow, 1988, 275 (in Russian).
Y. Takahashi, Ts. Ito, S. Sakai, and Y. Ishii, Chem. Commun., 1970, 1065.
J. B. Stothers, Carbon-13 NMR Spectroscopy, Academic Press, New York—London, 1972.
W. Bremser, L. Ernst, B. Franke, R. Gerhards, and A. Hardt, Carbon-13 NMR Spectral Data, 4th ed., Verlag Chemie, 1987, Spectrum No. 36303.
S. G. Davies, C. J. Goodwin, D. Pyatt, and A. D. Smith, J. Chem. Soc., Perkin Trans. 1, 2001, 1413.
Author information
Authors and Affiliations
Additional information
Dedicated to the memory of Academician V. A. Koptyug on the occasion of the 75th anniversary of his birth.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 6, pp. 1038–1044, June, 2006.
Rights and permissions
About this article
Cite this article
Osadchii, S.A., Shul’ts, E.E., Polukhina, E.V. et al. Study of alkaloids of the Siberian and Altai flora. Russ Chem Bull 55, 1077–1084 (2006). https://doi.org/10.1007/s11172-006-0380-2
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-006-0380-2