Abstract
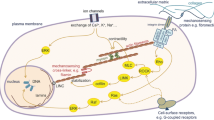
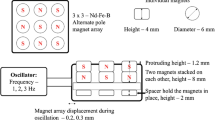
Nanoparticle entry into the cell depends on the surface charge and also on their size. Here, we report the entry of large magnetic nanoparticles (500 nm mean diameter) into the cell, being mediated by a mechanical stimulus supplied to the culture flasks. Investigations were carried out at 2–10 Hz frequency range with the vertical excursions ranging from 5 to 20 nm. Mechanical stimulation was found to aid the entry of both positive and negatively charged nanoparticles over a frequency range of 2–10 Hz. Transmission electron microscopy analysis indicated that, the stimulated samples could possibly mediate particle uptake through membrane invaginations, while the control samples indicated particles at the cell periphery, just outside the cell membrane. Mechanical stimulation had no significant effect on the cell morphology. Bromodeoxyuridine incorporation resulted in an increase in the proportion of S-phase in the stimulated samples compared with the controls, suggesting a reduction in the cell cycle duration. Mechanical stimulation could very well extend its effects to nanoscale cellular movements, and also facilitate the entry of large magnetic nanoparticle. This could be an interesting prospect for nanoparticle mediated drug delivery.







Similar content being viewed by others
References
Berry CC, Charles S, Wells S, Dalby MJ, Curtis ASG (2004) The influence of transferrin stabilised magnetic nanoaprticles on human dermal fibroblasts in culture. Int J Pharm 269:211–225. doi:10.1016/j.ijpharm.2003.09.042
Boccafoschi F, Bosetti M, Sandra PM, Leigheb M, Cannas M (2010) Effects of mechanical stress on cell adhesion: a possible mechanism for morphological changes. Cell Adh Migr 4(1):19–25. doi:10.4161/cam.4.1.9569
Brown TD (1995) Techniques for cell and tissue culture mechanostimulation: historical and contemporary design considerations. Iowa Orthop J 15:112–117
Brown TD (2000) Techniques for mechanical stimulation of cells in vitro: a review. J Biomech 33:3–14. doi:10.1016/S0021-9290(99)00177-3
Curtis ASG, Seehar GM (1978) The control of cell division by tension or diffusion. Nature 274:52–53. doi:10.1038/274052a0
Häfeli UO, Riffle JS, Harris-Shekhawat L, Carmichael-Baranauskas A, Mark F, Dailey JP, Bardenstein D (2009) Cell uptake and in vitro toxicity of magnetic nanoparticles suitable for drug delivery. Mol Pharm 6(5):1417–1428. doi:10.1021/mp900083m
Harush-Frenkel O, Debotton N, Benita S, Altschuler Y (2007) Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem Biophys Res Commun 353:26–32. doi:10.1016/j.bbrc.2006.11.135
Hughes S, Dobson J, Haj AE (2003) Mechanical stimulation of calcium signaling pathways in human bone cells using ferromagnetic micro-particles: implications for tissue engineering. Eur Cells Mater 6(2):43
Kim M, Javed NH, Yu JG, Christofi F, Cooke HJ (2001) Mechanical stimulation activates Galphaq signaling pathways and 5-hydroxytryptamine release from human carcinoid BON cells. J Clin Invest 108(7):1051–1059. doi:10.1172/JCI12467
Lange E, Koenig FO (1993) Handbuch der experimentalphysik. Leipzig, 263 pp
Li Y, Chen X, Gu N (2008) Computational Investigation of Interaction between nanoparticles and membranes: hydrophobic/hydrophilic effect. J Phys Chem B 112:16647–16653. doi:10.1021/jp8051906
Marchal G, Van Hecke P, Demaerel P, Decrop E, Kennis C, Baert AL, van der Schueren E (1989) Detection of liver metastases with superparamagnetic iron oxide in 15 patients: results of MR imaging at 1.5 T. Am J Roentgen 152:771–775. doi:0361-803X/AJR89/1524-07710
Overbeek JTHD (1952) Electrochemistry of the double layer, Chap 6, vol 1. In: Kruyt HR (ed) Colloid science. Elsevier, Amsterdam, pp 124–126
Petri-Fink A, Chastellain M, Juillerat-Jeanneret L, Ferrari A, Hofmann H (2005) Development of functionalized superparamagnetic iron oxide nanoparticles for interaction with human cancer cells. Biomaterials 26:2685–2694. doi:10.1016/j.biomaterials.2004.07.023
Pioletti DP, Muller J, Rakotomanana LR, Corbeil J, Wild E (2003) Effect of micromechanical stimulations on osteoblasts: development of a device simulating the mechanical situation at the bone-implant interface. J Biomech 36(1):131–135. doi:10.1016/S0021-9290(02)00301-9
Sahoo SK, Labhasetwar V (2003) Nanotech approaches to drug delivery and imaging. Drug Discov Today 8(24):1112–1120. doi:10.1016/S1359-6446(03)02903-9
Sahoo SK, Parveen S, Panda JJ (2007) The present and future of nanotechnology in human health care. Nanomed Nanotechnol Biol Med 3:20–31. doi:10.1016/j.nano.2006.11.008
Smith CA, de la Fuente J, Pelaz B, Furlani EP, Mullin M, Berry CC (2010) The effect of static magnetic fields and tat peptides on cellular and nuclear uptake of magnetic nanoparticles. Biomaterials 31:4392–4400. doi:10.1016/j.biomaterials.2010.01.096
Tripathi SC, Kerr J (1989) Effect of mechanical stress on cellular morphology. Tissue Cell 21(5):747–752. doi:10.1016/0040-8166(89)90083-9
Villanueva A, Canete M, Roca AG, Calero M, Veintamillas-Verdaguer S, Serna CJ, et.al. (2009) The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology 20(11):115103. doi: 10.1088/0957-4484/20/11/115103
Zentner A, Wieschollek JH, Heaney TG (2001) Effects of mechanical stimulation on cell cycle duration in rat gingival fibroblast progenitor cells. Eur J Oral Sci 109(4):267–272. doi:10.1034/j.1600-0722.2001.00062.x
Zhang LW, Monteiro-Riviere NA (2009) Mechanisms of quantum dot nanoparticle cellular uptake. Toxicol Sci 110:138–155. doi:10.1093/toxsci/kfp087
Acknowledgment
The authors wish to thank Carol-Anne Smith and Andrew Hart for their valuable technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaidyanathan, R., Curtis, A. & Mullin, M. Entry of large nanoparticles into cells aided by nanoscale mechanical stimulation. J Nanopart Res 13, 5301–5309 (2011). https://doi.org/10.1007/s11051-011-0516-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-011-0516-7