Abstract
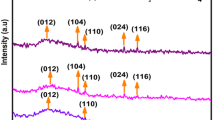
Nanoparticles of magnetite (Fe3O4) and hematite (α-Fe2O3) have been prepared by a simple microwave heating method using FeCl3, polyethylene glycol and N2H4·H2O. The amount of N2H4·H2O has an effect on the final phase of Fe3O4. The morphology of α-Fe2O3 was affected by the heating method. Crystalline α-Fe2O3 agglomerates were formed immediately at room temperature and most of these nanoparticles within agglomerates show the same orientation along [110] direction. After microwave heating, ellipsoidal α-Fe2O3 nanoparticles were formed following an oriented attachment mechanism. Both Fe3O4 and α-Fe2O3 nanoparticles exhibit a small hysteresis loop at room temperature.
Similar content being viewed by others
References
Adachi M., Murata Y., Takao J., Jiu J., Sakamoto M. and Wang F., (2004). Highly efficient dye-sensitized solar cells with a titania thin-film electrode composed of a network structure of single-crystal-like TiO2 nanowires made by the “Oriented Attachment” mechanism. J. Am. Chem. Soc. 126: 14943–14949
Alivisatos A.P., (2000). Naturally aligned nanocrystals. Science 289: 736–737
Banfield J.F., Welch S.A., Zhang H.Z., Ebert T.T. and Penn R.L., (2000). Aggregation-based crystal growth and microstructure development in natural iron oxyhydroxide biomineralization products. Science 289: 751–754
Bensebaa F., Zavaliche F., L’Ecuyer P., Cochrane R.W. and Veres T., (2004). Micorwave synthesis and characterization of Co-ferrite nanoparticles. J. Colloid Interf. Sci. 277: 104–110
Caillot T., Aymes D., Stuerga D., Viart N. and Pourroy G., (2002). Microwave flash synthesis of iron and magnetite particles by disproportionation of ferrous alcoholic solutions. J. Mater. Sci. 37: 5153–5158
Cheng F.Y., Su C.H., Yang Y.S., Yeh C.S., Tsai C.Y., Wu C.L., Wu M.T. and Shieh D.B., (2005). Characterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applications. Biomaterials 26: 729–738
Cheng Y., Wang Y.S., Chen D.Q. and Bao F., (2005). Evolution of single crystalline dendrites from nanoparticles through oriented attachment. J. Phys. Chem. B 109: 794–798
Deshpande K., Mukasyan A. and Varma A., (2004). Direct synthesis of iron oxide nanopowders by the combustion approach: reaction mechanism and properties. Chem. Mater. 16: 4896–4904
Goya G.F., Veith M., Rapalavicuite R., Shen H. and Mathur S., (2005). Thermal hysteresis of spin reorientation at Morin transition in alkoxide derived hematite nanoparticles. Appl. Phys. A-Mater. 80: 1523–1526
Jana N.R., Chen Y.F. and Peng X.G., (2004). Size- and shape-controlled magnetic (Cr, Mn, Fe, Co, Ni) oxide nanocrystals via a simple and general approach. Chem. Mater. 16: 3931–3935
Jia Z.B., Wei Y. and Wang H.M., (2000). Preparation of ultrafine hematite particles by microwave heating of ferric salt solution. J. Inorg. Mater. 15: 926–928
Jiang Y. and Zhu Y.J., (2005). Microwave-assisted synthesis of sulfide M2S3 (M = Bi, Sb) nanorods using an ionic liquid. J. Phys. Chem. B 109: 4361–4364
Jing Z.H., Han D.Z. and Wu S.H., (2005). Morphological evolution of hematite nanoparticles with and without sufactant by hydrothermal method. Mater. Lett. 59: 804–807
Josephson L., Tsung C.H., Moore A. and Weissleder R., (1999). High-efficiency intracellular magnetic labeling with novel superparamagnetic-tat peptide conjucates. Bioconjugate Chem. 10: 186–191
Katsuki H. and Komarneni S., (2001). Microwave-hydrothermal synthesis of monodispersed nanophase alpha-Fe2O3. J. Am. Ceram. Soc. 84: 2313–2317
Katsuki H., Shiraishi A., Komarneni S., Moon W.J., Toh S. and Kaneko K., (2004). Rapid synthesis of monodispersed alpha-Fe2O3 nanoparticles from Fe(NO3)3 solution by microwave irradiation. J. Ceram. Soc. Jpn. 112: 384–387
Lee D.K. and Kang Y.S., (2004). New synthetic method of magnetite nanocrystallites using γ-irradiation. Mol. Cryst. Liq. Cryst. 424: 85–94
Li Q. and Wei Y., (1998). Study on preparing monodispersed hematite nanoparticles by microwave-induced hydrolysis of ferric salts solution. Mater. Res. Bull. 33: 779–782
Liao X., Zhu J.J., Zhong W. and Chen H., (2001). Synthesis of amorphous Fe2O3 nanoparticles by microwave irradiation. Mater. Lett. 50: 341–346
Lopez-Quintela M.A. and Rivas J., (1993). Chemical-reactions in microemulsions- a powerful method to obtain ultrafine particles. J. Colloid Interf. Sci. 158: 446–451
Mansur H.S., Oréfice R.L. and Mansur A., (2004). Characterization of poly(vinyl alcohol)/poly(ethylene glycol) hydrogels and PVA-derived hybrids by small-angle X-ray scattering and FTIR spectroscopy. Polymer 45: 7193–7202
McMichael R.D., Shull R.D., Swartzendruber L.J., Bennett L.H. and Watson R.E., (1992). Magnetocaloric effect in superparamagnets. J. Magn. Magn. Mater. 111: 29–33
Mikhaylova M., Kim D.K., Bobrysheva N., Osmolowsky M., Semenov V., Tsakalakos T. and Muhammed M., (2004). Superparamagnetism of magnetite nanoparticles: dependence on surface modification. Langmuir 20: 2472–2477
Muench G.J., Arajs S. and Matijević E., (1981). Magnetic properties of monodispersed submicromic α-Fe2O3 particles. J. Appl. Phys. 52: 2493–2495
Namboodiri V.V. and Varma R.S., (2001). Microwave-accelerated Suzuki cross-coupling reaction in polyethylene glycol (PEG). Green Chem. 3: 146–148
Ocana M., Morales M. and Serna C.J., (1995). The growth-mechanism of alpha-Fe2O3 ellipsoidal particles in solution. J. Colloid Interf. Sci. 171: 85–91
Palchik O., Felner I., Kataby G. and Gedanken A., (2000). Amorphous iron oxide prepared by microwave heating. J. Mater. Res. 15: 2176–2181
Patnaik P., 2002. Handbook of Inorganic Chemistry. (ISBN 0-07-049439-8), the McGraw-Hill Companies, 344.
Penn R.L. and Banfield J.F., (1998). Imperfect oriented attachment: dislocation generation in defect-free nanocrystals. Science 281: 969–971
Penn R.L. and Banfield J.F., (1999). Morphology development and crystal growth in nanocrystalline aggregates under hydrothermal conditions: insights from titania. Geochem. Cosmochim. Acta 63: 1549–1557
Rigneau P., Bellon K., Zahreddine I. and Stuerga D., (1999). Microwave flash-synthesis of iron oxides nanoparticles. Eur. Phys. J. 7: 41–43
Schwertmann U., Friedl J. and Stanjek H., (1999). From Fe(III) ions to ferrihydrite and then to hematite. J. Colloid Interf. Sci. 209: 215–223
Shafi K.V.P., Ulman A., Dyal A., Yan X.Z., Yang N.L., Estourne’s C., Fourne’s L., Wattiaux A., White H. and Rafailovich M., (2002). Magnetic enhancement of γ-Fe2O3 nanoparticles by sonochemical coating. Chem. Mater. 14: 1778–1787
Saravanan L. and Subramanian S., (2005). Surface chemical studies on the competitive adsorption poly(ethylene glycol) and ammonium poly (methacrylate) onto of alumina. J. Colloid Interf. Sci. 284: 363–377
Si S.F., Li C.H., Wang X., Yu D.P., Peng Q. and Li Y.D., (2005). Magnetic monodisperse Fe3O4 nanoparticles. Cryst. Growth Des. 5: 391–393
Wang J., Chen Q., Zeng C. and Hou B.Y., (2004). Magnetic-field-induced growth of single-crystalline Fe3O4 nanowires. Adv. Mater. 16: 137–140
Wang W.W. and Zhu Y.J., (2004). Shape-controlled synthesis of zinc oxide by microwave heating using an imidazolium salt. Inorg. Chem. Commun. 7: 1003–1005
Wang W.W. and Zhu Y.J., (2005). Synthesis of PbCrO4 and Pb2CrO5 rods via a microwave-assisted ionic liquid method. Cryst. Growth Des. 5: 505–507
Wang W.Z., Zhan Y.J. and Wang G.H., (2001). One-step, solid-state reaction to the synthesis of copper oxide nanorods in the presence of a suitable sufactant. Chem. Commun. 8: 727–728
Yin M., Willis A., Redl F., Turro N.J. and O’Brien S.P. (2004). Influence of capping groups on the synthesis of gamma-Fe2O3 nanocrystals. J. Mater. Res., 19: 1208–1215
Zboril R., Mashlan M. and Petridis D., (2002). Iron(III) oxides from thermal processes-synthesis, structural and magnetic properties, Mössbauer spectroscopy characterization, and applications. Chem. Mater. 14: 969–982
Zhou Z.H., Wang J., Liua X. and Chanb H.S.O., (2001). Synthesis of Fe3O4 nanoparticles from emulsions. J. Mater. Chem. 11: 1704–1709
Zhu Y.J., Wang W.W., Qi R.J. and Hu X.L., (2004). Microwave-assisted synthesis of single-crystalline tellurium nanorods and nanowires in ionic liquids. Angew. Chem. Int. Ed. 43: 1410–1414
Acknowledgments
Financial support from National Natural Science Foundation of China (50472014) and Chinese Academy of Sciences under the Program for Recruiting Outstanding Overseas Chinese (Hundred Talents Program) is gratefully acknowledged. We thank the Fund for Innovation Research from Shanghai Institute of Ceramics, Chinese Academy of Sciences and the Natural Science Foundation from Science and Technology Committee of Shanghai (03ZR14104), P. R. China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, WW., Zhu, YJ. & Ruan, ML. Microwave-assisted synthesis and magnetic property of magnetite and hematite nanoparticles. J Nanopart Res 9, 419–426 (2007). https://doi.org/10.1007/s11051-005-9051-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-005-9051-8