Abstract
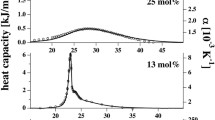
This article reports the influence of the preservative, propyl paraben (PPB), on the phase transition and dynamics of dipalmitoyl phosphatidylethanolamine (DPPE) vesicles both in multilamellar vesicular (MLV) and unilamellar vesicular (ULV) forms using DSC and (1H and 31P) NMR. DSC results indicate that the mechanism by which PPB interacts with DPPE vesicles is similar in both forms. Addition of PPB to DPPE dispersion results in lowering of the gel to liquid crystalline phase transition temperature (T m) and consequently increases DPPE headgroup fluidity. At high PPB concentration, additional transitions are observed whose intensity increases with increasing PPB concentration. DSC and NMR data indicate that the PPB molecules get intercalated between the DPPE headgroups as the polar group of the PPB molecules interacts with the polar group of PE, and the alkyl chain of PPB penetrates into the acyl chain region. The interesting finding with MLV is that the gel phase of DPPE in the presence of PPB, on equilibration at 25 °C, transforms to a stable crystalline subgel phases and whose intensity increases with increasing PPB concentration. The effect of inclusion of cholesterol in the PPB-free and PPB-doped DPPE dispersion was also studied.









Similar content being viewed by others
References
Soni MG, Burdock GA, Taylor SL, Greenberg NA. Safety assessment of propyl paraben: a review of the published literature. Food Chem Toxicol. 2001;39:513–32.
Elder RL. Final report on the safety assessment of methylparaben, ethylparaben, propylparaben, and butylparaben. J Am Coll Toxicol. 1984;3:147–209.
Perlovich GL, Rodionov SV, Bauer-Brandl A. Thermodynamics of solubility, sublimation and solvation processes of parabens. Eur J Pharm Sci. 2005;24:25–33.
Eklund T. Inhibition of growth and uptake processes in bacteria by some chemical food preservatives. J Appl Bacteriol. 1980;48:423–32.
Eklund T. The effect of sorbic acid and esters of p-hydroxybenzoic acid on the protonmotive force in Escherichia coli membrane vesicles. J Gen Microbiol. 1985;131:73–6.
Freese E, Levin BC. Action mechanisms of preservatives and antiseptics. Dev Incl Microbiol. 1978;19:207–27.
Freese E, Shen CW, Galliers E. Function of lipophilic acids as antimicrobial food additives. Nature. 1973;241:321–4.
Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004;24:1–4.
Darbre PD. Environmental oestrogens, cosmetics and breast cancer. Best Practice Res Clin Endocrinol Metabol. 2006;20:121–43.
Gennis RB. Biomembranes: molecular structure and function. New York: Springer; 1989.
Panicker L. Effect of propyl paraben on the dipalmitoyl phosphatidic acid vesicles. J Colloid Interface Sci. 2007;311:407–16.
Panicker L. Interaction of propyl paraben with dipalmitoyl phosphatidylcholine bilayer: a differential scanning calorimetry and nuclear magnetic resonance study. Colloids Surf B Biointerfaces. 2008;61:145–52.
Panicker L, Narasimhan SL, Mishra KP. Reduced fluidity of dipalmitoyl phosphatidic acid membranes by salicylic acid. Thermochim Acta. 2005;432:41–6.
Panicker L, Mishra KP. Salicylic acid-induced effects in the mixed-lipid (dipalmitoyl phosphatidylcholine–dipalmitoyl phosphatidylethanolamine) model membrane. J Colloid Interface Sci. 2005;290:250–8.
Panicker L, Mishra KP. Nuclear magnetic resonance and thermal studies on the interaction between salicylic acid and model membranes. Biophys Chem. 2006;120:15–23.
Koynova R, Caffrey M. Phases and phase transitions of the hydrated phosphatidylethanolamines. Chem Phys Lipids. 1994;69:1–34.
Stümpel J, Harlos K, Eibl H. Charge-induced pretransition in phosphatidylethanolamine multilayers. The occurrence of ripple structures. Biochim Biophys Acta. 1980;599:464–72.
Panicker L. Influence of the leprosy drug, dapsone on the model membrane dipalmitoyl phosphatidylethanolamine. Thermochim Acta. 2006;447:123–30.
Ladbrooke BD, Williams RM, Chapman D. Studies on lecithin-cholesterol-water interactions by differential scanning calorimetry and X-ray diffraction. Biochim Biophys Acta. 1968;29(150):333–40.
Oldfield E, Chapman D. Dynamics of lipids in membranes: heterogeneity and the role of cholesterol. FEBS Lett. 1972;23:285–97.
Tenchov BG, Lis LJ, Quinn PJ. Structural rearrangements during crystal–liquid–crystal and gel–liquid–crystal phase transitions in aqueous dispersions of dipalmitoylphosphatidylethanolamine. A time-resolved X-ray diffraction study. Biochim Biophys Acta. 1988;942:305–14.
Vaughan DJ, Keough KM. Changes in phase transition of phosphatidylethanolamine—and phosphatidylcholine—water dispersions induced by small modifications in the head group and backbone region. FEBS Lett. 1974;47:158–61.
Mantsch HH, Hsi SC, Butler KW, Cameron DG. Studies on the thermotropic behavior of aqueous phosphatidylethanolamines. Biochim Biophys Acta. 1983;728:325–30.
Horniak L, Kutejova E, Balgavy P. Effect of phase transitions in hydrated 1,2-dipalmitoylphosphatidylethanolamine bilayers on the spin probe order parameter. FEBS Lett. 1987;224:283–6.
Lin BZ, Yin CC, Hauser H. The effect of positive and negative pH-gradients on the stability of small unilamellar vesicles of negatively charged phospholipids. Biochim Biophys Acta. 1993;1147:237–44.
Tenchov B, Koynova R, Rapp G. New ordered metastable phases between the gel and subgel phases in hydrated phospholipids. Biophys J. 2001;80:1873–90.
Lewis RNAH, McElhaney RN. Calorimetric and spectroscopic studies of the polymorphic phase behavior of a homologous series of n-saturated 1, 2-diacyl phosphatidylethanolamine. Biophys J. 1993;64:1081–96.
Kodama M, Inoue H, Tsuchida Y. The behavior of water molecules associated with structural changes in phosphatidylethanolamine assembly as studied by DSC. Thermochim Acta. 1995;266:373–84.
Mulukutla S, Shipley GG. Structure and thermotropic properties of phosphatidylethanolamine and its N-methyl derivatives. Biochemistry. 1984;23:2514–9.
Chang H, Epand RM. The existence of a highly ordered phase in fully hydrated dilauroylphosphatidylethanolamine. Biochim Biophys Acta. 1983;728:319–24.
Seddon JM, Harlos K, Marsh D. Metastability and polymorphism in the gel and fluid bilayer phases of dilauroylphosphatidylethanolamine. Two crystalline forms in excess water. J Biol Chem. 1983;258:3850–4.
Wilkinson DA, Nagle JF. Metastability in the phase behavior of dimyristoylphosphatidylethanolamine bilayers. Biochemistry. 1984;23:1538–41.
Hentschel MP, Braun S, Dietrich R, Trahms L. NMR and X-Ray investigation of the phase behavior of phosphatidylethanolamines. Mol Cryst Liq Cryst. 1985;124:205–17.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panicker, L. Propyl paraben-induced changes in dipalmitoyl phosphatidylethanolamine vesicles. J Therm Anal Calorim 99, 583–592 (2010). https://doi.org/10.1007/s10973-009-0609-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0609-z