Abstract
Isotactic polypropylene(PP)/glass fiber(GF) composites were modified by grafting polymerization of polyfunctional monomer, pentaerythritol triacrylate (PETA), in the presence of 2,5-dimethyl-2,5-di(tert-butylperoxy) hexane peroxide (DDHP) via melt extrusion. Fourier transform infrared spectroscopy (FTIR), melt strength test (MS), mechanical property test, differential scanning calorimetry (DSC) and X-ray diffraction (XRD) were used to characterize the microstructure and properties of the modified composites. The crystallization kinetics was investigated by Mo method while apparent activation energy of crystallization of the composites was determined by Kissinger method. The FTIR results showed that the acrylic polymers were grafted onto the polypropylene chains. The grafting made the melt strengths and the mechanical properties of the modified composites, and the interfacial adhesion between PP and glass fiber all enhanced. High melting and crystallization temperatures, high crystallization rate and large activation energy of crystallization were also obtained after grafting. In addition, the grafted acrylic polymers recovered the depressed crystallization of polypropylene and restrained α-β transition in fatigue experiment.
Similar content being viewed by others
Introduction
Isotactic polypropylene (PP) is one of the most widely used commodity polymers. It has many desirable properties such as low density, high melting point and chemical resistance [1]. These characteristics make it become a polymer of a variety of general purpose and engineering applications after proper reinforcing modification, such as, the addition of either mineral or fibrous reinforcements [2]. For engineering applications, glass fiber is widely added into PP compounds to enhance the mechanical properties.
It is well known that, PP, non-polar and crystalline, exhibits very poor compatibility and adhesion toward other materials such as polymers, metals and inorganic fillers [3]. So the mechanical properties of the PP/GF composites are not much satisfied. The improvement of the interfacial adhesion between glass fibers and PP is necessary for the enhancement of mechanical properties of the composites. Many studies have been performed to better the adhesion between the fiber and matrix polymers [4–11]. The fiber modification was done by using aminosilane coupling agent [5], silane treatment [6], copolymer coupling agent [7, 8] and the likes. On the other hand, polymer matrix was often chemically modified with functional monomers such as maleic anhydride [9–11].
High melt strength PP has shown superior processability especially for extension and foaming processes. The research on the grafting long-branch chains for high melt strength PP has been intensively made in the past decade [12–16]. Many researchers prepared long-chain branched PP via grafting a variety of polyfunctional monomers, such as trimethylol propane triacrylate [12], diethylene glycol diacrylate, and pentaerythritol triacrylate (PETA) [13–16]. Kim et al. [13] concluded that triacrylates were the most effective among the polyfunctional monomers used. Wang et al. [14] produced long-chain branched PP by a reactive extrusion process using a mixture of PETA and 2,5-dimethyl-2,5-di(tert-butylperoxy) hexane peroxide (DDHP) and the flow curves and thermal properties of the resulted polypropylenes were investigated. They suggested that low concentrations of polyfunctional monomer and peroxide be used in order to minimize the formation of macrogels. Above all, the grafting of polyfunctional monomer onto the polypropylene skeletons can not only increase the melt strength of PP, but also change its non-polarity.
In this work, we focused on studying the processability and applicability of the PP/glass fiber composite for large diameter paper mandrel pipe in papermaking industry. In fact, the processing, the mechanical properties and the mechanical stability of the mandrel pipe during service haven’t ever been studied as far. These concerns were handled with the grafting polymerization of polyfunctional monomer, PETA and the reinforcing modification of chopped glass fiber during extrusion process. The mechanical stability via checking α-β transition and the change of crystallinity were examined by fatigue experiment, and the microstructure and other properties of the resulted PP/glass fiber composite grafted with branching polyacrylics were studied with FTIR, SEM, DSC and XRD.
Experimental
Raw materials
The isotactic polypropylene (PP) and glass fiber were kindly supplied by Sunway Polymer Co. Ltd, Suzhou, China. The initiator, DDHP was obtained from Akzo Nobel Decorative Coatings (Guangzhou) Co. Ltd, China. Pentaerythritol triacrylate (PETA) was procured from Dongjiao Chemical Plant, Liyang, Jiangsu, China. Irganox 1010, pentaerythrite tetra[β-(3,5-ditertbutyl-4-hydroxyphenyl)-propionate] was gifted from Sunway Polymer Co. Ltd, Suzhou, China. These products were used without further purification.
Sample preparation
The basic formulation for the composite pipe is as follows: PP, 2,125 g; GF, 375 g; Irganox 1010, 5 g; Peroxide, 0.5 g. The amount of polyfunctional monomer, PETA, was 0, 1 wt%, 3 wt%, 5 wt% and 7 wt% based on the total weight of the above formulation, which was labeled as A1, A2, A3, A4 and A5 respectively. DDHP and PETA were first dissolved in acetone separately, and then the solutions and Irganox 1010 were added into the mixture of glass fiber and PP. Finally the mixture was stirred for 5 min in a high speed mixer and placed in ventilating cabinet at room temperature until the acetone volatilized completely. The grafting polymerization took place in a twin-screw extruder at die temperature of 190 °C at a screw rotating speed of 110 rpm. The extrudates were cooled in water and then pelletized.
Characterization
Grafting
Fourier transform infrared resonance was used to examine the grafting polymerization of PETA. The pelletized chips mentioned above were dissolved in xylene under reflux and the supernatant of the resultant solutions were precipitated with acetone, and the precipitants were used for grafting analysis with AVATAR 360 spectrometer (Nicolet, USA).
Mechanical properties
The samples for mechanical tests were injection molded and the tests were carried out by following the ISO standards. The tensile (ISO178) and flexural (ISO527) tests were done with Electronic Universal Testing Machine (ZWICK Z010) at the rate of 50 mm/min, and the Izod notched impact test (ISO178) was realized with Pendulum-type Impact Tester (ZWICK B5113.300). The above tests were done at room temperature, and at least three measurements for each sample were made to report the average.
Morphology
The injection molded samples were placed in liquid nitrogen and then fractured. The fractured cross-section morphology of the samples was observed by scanning electron microscope (S-4700, Hitachi, Japan) after being gold sputtered. The scanning voltage was 15.0 kV.
Crystallization
The melting and crystallization behaviors of the samples were carried out with a differential scanning calorimeter (SDT 2960, DSC 2010, TA instruments, USA). All runs were carried out under nitrogen atmosphere at a flow rate of 40 ml/min to prevent oxidation. The procedure was as follows: the samples (about 1.5 mg) were heated to 250 °C and maintained for 5 min to remove thermal history; then the sample was cooled down to 50 °C and its crystallization behaviour was recorded. Finally the sample was heated again up to 250 °C and its melting behaviour was recorded as well. Both the heating and cooling rates were fixed at 10 °C/min. The crystallization temperature (T c) and the melting temperature (T m) of the sample are listed in Table 1.
The procedure for non-thermal crystallization was as follows: all samples were heated to 300 °C at a rate of 20 °C/min and held in the molten state for 5 min to eliminate thermal history, and then the samples were cooled down to 50 °C at constant rates of 5 °C/min, 10 °C/min, 15 °C/min, and 20 °C/min, respectively. The exothermic curves of heat flow as a function of temperature were recorded.
Processability
While the molten polypropylene was extruded from the orifice of the melt indexer, the diameter of the extrudate was gradually changed due to extrusion swelling of polymers. By measuring the change in diameter of the extruded cord, the melt viscosity can be calculated. The calculated viscosity was named as melt strength (MS) since it indicates how well the melt can support its own weight. The equation used to calculate the melt strength (in poises) is [17],
Where, ∆l is the length of the cord (in inch) required for a 50% decrease in diameter of the cord, r 0 is the radius (in inch) of the cord as it first emerges from the die (measured by extrapolation), and MFI230 is the melt flow index in g/10 min at 230 °C with the load of 2.16 kg on the melt indexer.
Fatigue
The injection molded samples (ISO527) were subjected to fatigue experiment on the Electronic Universal Testing Machine by using the user's procedure. The sample was loaded on the two jaws separated by 64 mm in the free beam mode, and the center position of the sample was displaced downwards 5 mm under load, and then the force was removed while moving upwards. The down and up cycle operation was repeated 200 times. After that, the loaded surface was changed upside down and repeated under load 200 times. Finally the center loaded area of the sample was cut and used for XRD measurement. The fatigued samples were labeled as F1, F2, F3, F4 and F5. Afterwards, XRD measurement was performed for the all samples at room temperature with X-Ray Diffractometer (X’ Pert PRO MRD, Panalytical, Holland). The data were collected from 12° to 24° with the resolution of 0.02°.
Results and discussion
Grafting of acrylics
The FTIR spectra of the samples A1, A2 and A3 are shown in Fig. 1. Compared with the sample A1, the samples A2 and A3 gave an adsorption band at about 1,735 cm−1, which can be assigned to the stretching vibration of the carbonyl group of the ester from the polyacrylic of PETA, indicating that PETA was grafted onto the PP skeleton. The intensity of the band at 1,735 cm−1 of the sample A3 was stronger than that of A2. It can be seen that the grafting degree increases with the concentration of PETA, suggesting that higher dosage of PETA would favor more abundant grafting.
Mechanical properties
The effect of PETA concentration on mechanical properties is shown in Fig. 2. There were obvious improvements on the tensile strength and modulus, flexural strength and modulus, impact strength of the glass fiber-reinforced PP composites after the grafting of PETA in comparison with those in the absence of PETA. In fact, tensile modulus, flexural strength and modulus were increased with the PETA concentration until 5 wt% whereas tensile strength and notched impact strength reached maximum at 3 wt% and 1 wt%, respectively. It seems that an excess amount of PETA worsens their mechanical properties.
The above phenomena can be explained as follows: non-polar PP normally presents very poor adhesion towards glass fiber, whereas the grafted polyacrylics on the polypropylene chains contributes some polarity for PP, and thus the grafting improves the interfacial adhesion between glass fiber and PP. The good interfacial interaction can promote stress transfer from the matrix polymer to the glass fibers while the samples are mechanically loaded. Finally the mechanical properties of PP/GF composites are improved. However, an excess of PETA would produce homopolymer, which acts as impurity in the PP/GF composite, and the interfacial adhesion between grafted PP and glass fiber was weakened due to the impurity, and thus the mechanical properties were aggravated again.
Morphology
The glass fiber-polypropylene interaction was analyzed by SEM studies of the fractured surfaces. The SEM images of the samples A1, A2, A3 and A5 are shown in Fig. 3. From the image of the sample A1, without PETA, many of the glass fibers were pulled out while fracturing, and the surfaces of the fibers were primarily smooth and clean, and the fractured polypropylene surfaces were also basically flat. These phenomena all indicate that the interfacial adhesion of PP/GF is very poor and polypropylene matrix is brittle. Compared with the sample A1, the glass fibers in the samples A2, A3 and A5 were only partially pulled out, and the surfaces of the fibers were stained with the grafted polymers as shown in the white arrows, and there were no any crevices between the fiber and the matrix polymer in the grafting cases as indicated in the black arrows, although the severe fractured action was once imposed. In addition, the dispersity of glass fiber in polypropylene matrix is getting better and the pulled length of the fibers is becoming shorter with the higher dosage of PETA. The fractured polypropylene surfaces also became rough with the increase of PETA. Conclusively, the grafted polypropylenes are enriched around the glass fibers and thus improve the dispersity of the glass fiber and the adhesion between the fiber and the matrix polymer. That’s why the mechanical properties of polypropylene/glass fiber composites are enhanced with the grafting of acrylic polymer .
Non-isothermal crystallization
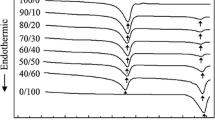
Figure 4 shows the melting and crystallization bebaviours of the samples A1–A5. The crystallization temperature (T c) and melting temperature (T m) are listed in Table 1. Both of T m and T c of the samples A2–A5 were all higher than those of A1, and this complied well with literature [16, 18]. The increases of T m are explained by the restricted melt flow due to the grafting of bulkily branched polyacrylic chains. The effect of restricted melt flow on crystallization temperature can be found in the Wang et al.’s work [19]. The increases of T c are accounted for by nucleation of the grafted polypropylene and thus acceleration of crystallization [16].
Most industrial processing proceeds under non-isothermal conditions, so that the study of non-isothermal crystallization is of great practical importance. The crystallization exotherms of the samples A1, A2 and A5 are shown in Fig. 5. The crystallization temperatures (T c) of all samples at different cooling rates are shown in Table 2. With the increase of cooling rate, the T c of all samples shifted to lower temperatures. The reason is that macromolecular chains can not enter crystal-grain within due time when sample temperature decreases too fast, so polymer crystallizes under much large degree of supercooling. The T c values of the samples A2–A5 were higher than that of A1 for each cooling rate. Therefore, it still can be concluded that the grafted polymer acts as nucleating agent to accelerate the crystallization process.
During the non-isothermal crystallization process, the relative crystallinity at any crystallization temperature X(t) can be obtained from the area ratio between integral area at temperature T and the whole crystallization area [20].
Where, T 0 is the onset crystallization temperature, \( {T_\infty } \) is the end crystallization temperature, and T is the temperature at time t.
The crystallization time (t) has the following relationship with T:
Where, Φ is the cooling rate.
Figure 6 is the plots of the relative crystallinity X(t) vs. crystallization time t for the samples A1, A3 and A4. All these curves had almost the same sigmoidal shape, implying the lag effect of cooling rate on crystallization. Zhang et al. [21] also reported the shape in their amino-terminated polyurethane grafted polypropylene, and they thought that, the first non-linear part was corresponded to nucleation stage, and the linear region implied primary crystallization, and the last non-linear region was the secondary crystallization. The half-time of crystallization (t 1/2), the time corresponding to 50% of the relative crystallinity, was obtained from Fig. 6 and is listed in Table 2. The half-time of crystallization directly indicates the rate of crystallization. The shorter t 1/2 is, and the faster crystallization becomes. The decrease of t1/2 values with the increase of cooling rates for all samples implies a progressively speeding up crystallization rate. Moreover, t1/2 values of the samples A2, A3, A4 and A5 were shorter than that of A1 at all cooling rates. The results suggest that grafting of PETA accelerate crystallization. Of course, an excess amount of PETA, here ca. 7 wt%, didn’t give the extra acceleration crystallization.
There are several different methods used to discuss the non-isothermal crystallization kinetics [22–25]. In this paper, the Mo method was used. Avrami model can be directly applied for non-isothermal crystallization kinetics of polymers [22]. According to the model, relative crystallinity develops with time as follows:
Where, X(t) is the relative crystallinity at crystallization time t; Z t is the crystallization rate constant; n is the Avrami exponent, which depends on the type of nucleation and the growth dimension.
Considering the character of non-isothermal crystallization, the parameter for the value of the crystallization rate, Z t, should be corrected by the cooling rate Φ. So Z c was introduced by Jeziorny [23]:
Considering the effect of a cooling (or heating) effect, Ozawa [24] extended the Avrami equation to develop a simple method to study the non-isothermal experiment. The general form of Ozawa theory is written as follows:
Where, K(T) is the cooling crystallization function; Φ the cooling rate and m the Ozawa exponent that depends on the dimension of the crystal growth.
Mo et al. [25] tried to describe the non-isothermal crystallization process precisely by combining the Avrami and Ozawa equations, they obtained the following equation:
Where, F(T), equal to [K(T)/Z t]1/m, is the value of cooling rate, which has to be chosen at unit crystallization time when the measured system has a certain degree of crystallinity [21], and α = n/m, the ratio between the Avrami and Ozawa exponents.
According to Eq. (8), the plots of lnΦ vs. lnt at different relative crystallinities of 20%, 40%, 60%, 80% and 90% are shown in Fig. 7. The parameters α and F(T) can be obtained from the slope and the intercept of the regression lines. The values of α and F(T) are also listed in Table 3. At a given crystallinity, a high value of F(T) means a high cooling rate needed to reach the crystallinity in a minute, which accordingly reflects the difficulty of its crystallization process. Table 3 shows that the value of F(T) increases with the increase of crystallinity while the values of α are almost constant. By comparing the values of F(T) for all the samples, it can be found that the values of F(T) for the sample A1 are generally higher than those for the samples A2–A5. This result indicates that the grafted polypropylene accelerates the crystallization of PP again. In fact, the crystallization rate is controlled by nucleation at early stage while heterogeneous nucleation accelerates the initial crystallization rate. However, when the samples A2–A5 are compared, the sample A5 doesn’t show any further acceleration crystallization again, and this may imply that excess PETA doesn’t graft onto polypropylene backbone any more. Conclusively, the equation of Mo method successfully describes the non-isothermal crystallization process of PP/GF modified by PETA and also distinguishes the grafting process.
Kissinger [26] proposed that the activation energy of crystallization be determined by calculating the variation of the crystallization peak temperature T c with the cooling rate Φ. Kissinger equation is
Where, R is the gas constant (8.314 kJ/(mol·k)), and ΔE is the activation energy of crystallization process. The graphs of \( \ln \left. {\left( {\Phi /T_c^2} \right)} \right] \) vs. 1/T c are shown in Fig. 8. The slope of the curve determines ΔE/R. The activation energy ΔE can be got and is shown in Table 3. Traditionally, the activation energy, the excess energy, should be infused into a reaction system in order to trigger a chemical reaction and is thus often positive value. In our case, in the polymer melt, all polymer chains have too much energy to quietly be folded into crystal nuclei, and the “activation energy”, the excess energy, should be deprived of from the polymer chains and thus the negative sign is assigned. Since the grafting of polyacrylics into polypropylene chains accelerates the nucleation and crystallization of polypropylene, more excessive energy from the grafted polymer system than from virgin polypropylene should be deprived of. That’s why the value of activation energy of grafted polypropylene is larger than that of pristine polypropylene.
Melt strength
In the production process, there always exist some extensions in the die flow and the extrudate flow from die to register ring, especially for the large diameter paper mandrel pipe. In fact, the mandrel pipe is expected to extend in the production process to some extent in order to counterbalance the thermal expansion since the pipe will encounter temperature rise from room temperature to ca. 75 °C during its service. Therefore, the melt extension flow is always desired for the product. Figure 9 shows the effect of PETA concentration on the melt flow index (MFI) and the melt strength (MS). The MFI values of the modified PP/GF composites decreased while the MS values increased with the increase of PETA content. The elevation of MS is consistent with the result of Wang et al. [14]. The increases of MS values were over 4 times when the dosage of PETA was 5 wt%, and thus the processability would be expected to be improved much.
Mechanical stability after fatigue
The polypropylene paper mandrel pipe to be developed will be endured both mechanical load and high frequency’s vibration load except for the temperature rise during its service, thus the fatigue resistance will be one of important engineering parameters. Unfortunately, PP normally has three polymorphic forms, α (monoclinic), β (pseudohexagonal), and γ(triclinic), with α-crystal being the most stable structure. Since these three crystals have different density and modulus, the α-β transformation and the change of crystallinity under dynamic and mechanical loads will certainly affect the service life expectance of the pipe in its engineering application.
The XRD patterns of the samples A1, A2, F1 and F2 are shown in Fig. 10. The strong diffraction peaks located at the scattering angles (2θ) of ca. 14.10, 16.90, 18.58, 21.10, 21.76 and 25.38° correspond to the reflections from α (110), α (040), α (130), α (111), and α (131) crystal planes respectively, while the peak located at the angle 2θ of 16.10 belongs to the reflection of β (300) crystal plane. The diffractograms indicated that the samples crystallized mainly in two crystal forms, α and β. It is quite often to have α crystals in the isotactic polypropylene whereas there seldom existed β crystals in literature after the modification with PETA grafting [16]. Tabatabaei et al. [18] and Ni et al. [27] even thought that, the addition of long-chain branched polypropylene to isotactic polypropylene could hamper the formation of β crystals, although there was trace of amount of γ crystals appeared. The existence of β crystals in our study may be explained as the experimental scale difference. Our samples were prepared on large scale and dynamic experiment, different from Zhou’s experimental setup (Haake rheometer, small scale and static) [16], and thus the melt mixing and grafting uniformities can’t be ensured completely. In fact, Lugão et al. also got β crystals during their irradiation branching polypropylene industrial production [28].
The degree of crystallinity of the α-form of PP can be obtained according to the equation [29]:
Where, W c,x is the degree of crystallinity of α-form PP; I a is the integral intensity of the amorphous peak; \( {I_{\rm{c}}} = {I_{110}} + 1.63{I_{040}} + 2.14{I_{130}} + 3.51{I_{041}} \); I 110, I 040, I 130 and I 041 are the integral intensities of corresponding crystalline peak.
The degrees of crystallinity of α-crystals for the samples A1–A5 and F1–F5 are listed in Table 4. The results indicated that, although the grafting of PETA accelerated the crystallization behaviour, the degrees of crystallinity of the grafted samples were all reduced for the samples A2–A5, as compared with the sample A1. In fact, the acceleration of crystallization should originate from the nucleation of grafting polypropylene and possibly also from large melt viscosity, which may afford the stability of crystalline nuclei at high temperatures. On the other hand, the high viscosity also suppresses crystallization due to the slowdown of the segmental transition at high temperatures, and this causes the reduction of the degree of crystallinity. However, the degrees of crystallinity of the fatigued samples F2–F5 were increased whereas that of the sample F1 was decreased. The reason for the different tendency for the samples F series can be that, the depressed crystallization for the samples A2–A5 is activated while the fully developed crystallite of the sample A1 is destroyed during the action of dynamic load. The idea can be indirectly testified by the countershifting of peak positions of the samples F1 and F2, namely the scattering angles of the sample F1 were all increased while those of the sample F2 all decreased in comparison with their counterparts, the samples A1 and A2. Based on the Bragg equation, the increase of scattering angle of (hkl) crystal plane implies the decrease of distance of (hkl) crystal plane. The change of distance of (hkl) crystal plane may signpost the change of crystallization stress in the crystal cell. Therefore, the decreases of scattering angles of all (hkl) crystal planes of the sample F2 indicate the release of crystallization stress and the increase of crystallinity, and vice versa for the sample F1. In addition, the increase of crystallinity, namely the increase of modulus, means the increase of deformation resistance for the samples F2–F5 under dynamic mechanical load, and vice versa for the sample F1.
The relative proportions of the β-form, k can be calculated according to the Turner-Jones and Cobbold equation [30]:
Where, \( {h_{\alpha 1}} \), \( {h_{\alpha 2}} \) and \( {h_{\alpha 3}} \) are the heights of the α-form diffraction peaks corresponding to the reflections of (110), (040), and (130) crystal planes, respectively, and h β is the height of the β-form (300) peak.
The results of the relative proportions of the β-form are listed in Table 5. The k of the sample A1 changed remarkably from 13.87% to 17.17% while the values of the samples A2–A5 were increased quite little. β-crystals are induced to be formed under the stress and strain actions. Since the modulus of β-crystals is lower than that of α-crystals, the increase of β-form content will cause the reduction of deformation resistance of the engineering pipe during its application. The results indicate that the grafted polypropylene has the effect on preventing the formation of β-crystals in fatigue experiment. Namely, the grafting of polyacrylics can make PP a bit of stable under cyclic strain action based on the increase of crystallization and the suppression of α-β transition to some extent.
Conclusions
-
(1)
Isotactic polypropylene(iPP)/glass fiber(GF) composites were modified by grafting poly(pentaerythritol triacrylate). The FTIR spectra suggest that the grafting degree increase with the concentration of PETA.
-
(2)
The interfacial adhesion between PP and glass fiber was improved after grafting. The melt strengths and the mechanical properties of grafted polypropylenes were heightened obviously to some extent.
-
(3)
The increases of crystallization and melting temperatures and of crystallization activation energy were attributed to the acceleration of crystallization process and the stability of crystal nuclei of the grafted polypropylene.
-
(4)
The grafted polypropylene recovers the depressed crystallization and restrains the α-β transition under cyclic strain load to some extent.
References
Langston J, Dong JY, Chung TC (2005) Macromolecules 38(14):5849–5853
Karian HG (ed) (2003) Handbook of polypropylene and polypropylene composites, 2nd edn. Marcel Dekker, New York
Yu J, He J (2000) Polymer 41:891–898
Morelli CL, Pouzada AS, Sousa JA (2009) J Appl Polym Sci 114:3592–3601
Nishitani Y, Sekiguchi I, Hausnerova B, Zdrazilova N, Kitano T (2007) Polym Compos 15(2):111–119
Felix AHO, Cardozo NSM, Nachtigall SMB, Mauler RS (2006) Macromol Mater Eng 291:418–427
Li Y, Chen LX, Zhou XD (2008) J Mater Sci 43(15):5083–5091
Zhou SH, Gao Y, Wang YT, Hu CP, Dong QZ (2007) J Appl Polym Sci 104:1661–1670
Kumar KS, Ghosh AK, Bhatnagar N (2007) J Reinf Plast Compos 26(3):239–249
Kumar KS, Ghosh AK, Bhatnagar N (2007) Polym Compos 28(2):259–266
Xie HQ, Zhang S, Xie D (2005) J Appl Polym Sci 96:1414–1420
Graebling D (2002) Macromolecules 35:4602–4610
Kim BK, Kim KJ (1993) Adv Polym Tech 12(3):263–269
Wang XC, Tzoganakis C, Rempel GL (1996) J Appl Polym Sci 61:1395–1404
Tian JH, Yu W, Zhou CX (2006) Polymer 47:7962–7969
Tian JH, Yu W, Zhou CX (2006) J Macromol Sci Part B Physics 45:969–985
Guillet JE, Combs RL, Slonaker DF, Weemes DA, Coover HW (1965) J Appl Polym Sci 8:757–765
Tabatabaei SH, Carreau PJ, Ajji A (2009) Chem Eng Sci 64:4719–4731
Wang Y, Run M (2009) J Polym Res 16:725–737
Avrami M (1940) J Chem Phys 8:212–224
Zhang Y, Jiang X, Guan Y, Zheng A (2005) Mater Lett 59:3626–3634
Dejuana R, Jauregui A, Calahorra E, Cortazar M (1996) Polymer 37:3339–3345
Jeziorny A (1978) Polymer 19:1142–1144
Ozawa T (1971) Polymer 12:150–158
Liu JP, Mo ZS, Qi YC, Zhang HF, Chen DL (1993) Acta Polym Sin 1:1–6, In Chinese
Kissinger HE (1956) J Res Natl Bur Stand 57:217–221
Ni QL, Fan JQ, Dong JY (2009) J Appl Polym Sci 114:2180–2194
Lugão AB, Cardoso E, Lima LF, Hustzler B, Tokumoto S (2003) Nucl Instrum Methods Phys Res Sect B 208:252–255
Ye CM, Liu JJ, Mo ZS, Tang GB, Jing XB (1996) J Appl Polym Sci 60:1877–1881
Turner-Jones A, Aizlewood JM, Beckett DR (1964) Makromol Chem 75:134–158
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ni, Q., Zhu, X., Wang, Y. et al. Microstructure and properties of polypropylene/glass fiber composites grafted with poly(pentaerythritol triacrylate). J Polym Res 18, 917–926 (2011). https://doi.org/10.1007/s10965-010-9489-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-010-9489-y