Abstract
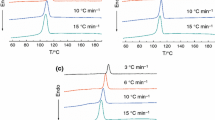
The crystallization behaviors, non-isothermal crystallization kinetics, and the morphology of poly(trimethylene terephthalate)/Polypropylene (PTT/PP) blends using a maleic anhydride grafted polypropylene (PP-g-MAH) as a compatibilizer were investigated by differential scanning calorimeter (DSC) and scanning electron microscope (SEM), respectively. The results suggested that the blends exhibited different crystallization and melting behaviors due to different content of PP-g-MAH. All of the DSC curves of the blends exhibited two exothermic peaks and endothermic peaks. The commonly used Avrami equation modified by Jeziorny, Ozawa theory and the method developed by Mo were used, respectively, to fit the primary stage of non-isothermal crystallization process. The results suggested that the crystallization rate of PTT component was increased, whereas, that of PP component was retarded with the introduction of PP-g-MAH. The effective activation energy was calculated by differential iso-conversional method developed by Vyazovkin. The SEM result suggested that the introduction of PP-g-MAH greatly improved the compatibility between PTT and PP, and decreased the size of dispersed particles.














Similar content being viewed by others
Reference
Whinfield JR, Dickson JT (1946) Brit Pat 578:079
Wu J, Schultz JM, Samon JM et al (2001) Polymer (Guildf) 42:7141. doi:10.1016/S0032-3861(01)00042-8
Grande JA (1997) Mod Plast 12:97
Li JX, Cheung WL, Jia D (1999) Polymer (Guildf) 40:1219. doi:10.1016/S0032-3861(98)00345-0
Coccorullo I, Pantani R, Titomanlio G (2003) Polymer (Guildf) 44:307. doi:10.1016/S0032-3861(02)00762-0
Masubuchi Y, Watanabe K, Nagatake W et al (2001) Polymer (Guildf) 42:5023. doi:10.1016/S0032-3861(00)00886-7
Assoulinea E, Pohl S, Fulchiron R et al (2000) Polymer (Guildf) 41:7843. doi:10.1016/S0032-3861(00)00113-0
Supaphol P, Lin JS (2001) Polymer (Guildf) 42:9617. doi:10.1016/S0032-3861(01)00507-9
Supaphol P, Spruiellb JE (2001) Polymer (Guildf) 42:699. doi:10.1016/S0032-3861(00)00399-2
Zhang Y, Jiang X, Guan Y et al (2005) Mater Lett 59:3626. doi:10.1016/j.matlet.2005.07.010
Supaphol P (2001) Thermochim Acta 370:37. doi:10.1016/S0040-6031(00)00767-X
Sekia M, Yamauchia S, Matsushitab Y (1999) J Phys Chem Solids 60:1333. doi:10.1016/S0022-3697(99)00120-1
Silvestre C, Cimmino S, Alma ED et al (1999) Polymer (Guildf) 40:5119. doi:10.1016/S0032-3861(98)00696-X
Shieh YT, Lee MS, Chen SA (2001) Polymer (Guildf) 42:4439. doi:10.1016/S0032-3861(00)00567-X
Rabello MS, White JR (1997) Polymer (Guildf) 38:6389. doi:10.1016/S0032-3861(97)00214-0
Albano C, Papa J, Ichazo M et al (2003) Compos Struct 62:291. doi:10.1016/j.compstruct.2003.09.028
Campoy I, Arribas JM, Zaporta MAM et al (1995) Eur Polym J 31:475. doi:10.1016/0014-3057(94)00185-5
Chen JH, Tsai FC, Nien YH et al (2005) Polymer (Guildf) 46:5680. doi:10.1016/j.polymer.2005.03.107
Xiao Z, Li L, Zhou D et al (2003) Thermochim Acta 404:283. doi:10.1016/S0040-6031(03)00186-2
Hieber CA (1995) Polymer (Guildf) 36:1455. doi:10.1016/0032-3861(95)95925-Q
Dangseeyun N, Supaphol P, Nithitanakul M (2004) Polym Test 23:187. doi:10.1016/S0142-9418(03)00079-5
Rwei SP (1999) Polym Eng Sci 39:2475
Ravikumar HB, Ranganathaiah C, kunavaswany GN et al (2005) Polymer 46:2372. doi:10.1016/j.polymer.2004.12.058
Zhang QX, Yu ZZ, Xie XL et al (2004) Polymer (Guildf) 45:5985. doi:10.1016/j.polymer.2004.06.044
Chan CM, Wu JS, Li JX et al (2002) Polymer (Guildf) 43:2981. doi:10.1016/S0032-3861(02)00120-9
Xu WB, Liang GD, Zhai HB et al (2003) Eur Polym J 39:1467. doi:10.1016/S0014-3057(03)00015-6
Xue ML, Yu YL, Chuah HH (2007) J Macromol Sci Phys 46:603. doi:10.1080/00222340701258008
Avarami M (1940) J Chem Phys 8:212. doi:10.1063/1.1750631
Jeziorny A (1978) Polymer (Guildf) 19:1142. doi:10.1016/0032-3861(78)90060-5
Ozawa T (1971) Polymer (Guildf) 12:150. doi:10.1016/0032-3861(71)90041-3
Liu TX, Mo ZS, Zhang HF (1998) J Polym Eng 18:283
Friedman H (1964–1965) J Polym Sci 6(Part C):183
Vyazovkin S (1997) J Comput Chem 18:393. doi:10.1002/(SICI)1096-987X(199702)18:3<393::AID-JCC9>3.0.CO;2-P
Vyazovkin S (2001) J Comput Chem 22:178. doi:10.1002/1096-987X(20010130)22:2<178::AID-JCC5>3.0.CO;2-#
Choe CR, Lee KH (1989) Polym. Eng Sci 29:801
Liu MY, Zhao QX, Wang YD et al (2003) Polymer (Guildf) 44:2537. doi:10.1016/S0032-3861(03)00101-0
Acknowledgment
The work is supported by the financial support from the Natural Science Foundation of Hebei Province (B2007000108), PR China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Run, M. Non-isothermal crystallization kinetic and compatibility of PTT/PP blends by using maleic anhydride grafted polypropylene as compatibilizer. J Polym Res 16, 725–737 (2009). https://doi.org/10.1007/s10965-009-9279-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-009-9279-6