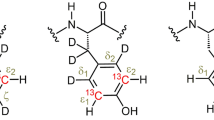
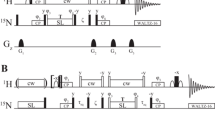
Abstract
Dynamics of large-amplitude conformational motions in proteins are complex and less understood, although these processes are intimately associated with structure, folding, stability, and function of proteins. Here, we use a large set of spectra obtained by cross-relaxation suppressed exchange NMR spectroscopy (EXSY) to study the 180° flipping motion of the Y97 ring of horse ferricytochrome c as a function of near-physiological temperature in the 288–308 K range. With rising temperature, the ring-flip rate constant makes a continuous transition from Arrhenius to anti-Arrhenius behavior through a narrow Arrhenius-like zone. This behavior is seen not only for the native state of the protein, but also for native-like states generated by adding subdenaturing amounts of guanidine deuterochloride (GdnDCl). Moderately destabilizing concentrations of the denaturant (1.5 M GdnDCl) completely removes the Arrhenius-like feature from the temperature window employed. The Arrhenius to anti-Arrhenius transition can be explained by the heat capacity model where temperature strengthens ground state interactions, perhaps hydrophobic in nature. The effect of the denaturant may appear to arise from direct protein-denaturant interactions that are structure-stabilizing under subdenaturing conditions. The temperature distribution of rate constants under different stability conditions also suggests that the prefactor in Arrhenius-like relations is temperature dependent. Although the use of the transition state theory (TST) offers several challenges associated with data interpretation, the present results and a consideration of others published earlier provide evidence for complexity of ring-flip dynamics in proteins.





Similar content being viewed by others
Abbreviations
- EXSY:
-
Cross-relaxation suppressed exchange NMR spectroscopy
- GdnDCl:
-
Guanidinium deuterochloride
- cyt:
-
Cytochrome
- ferricyt:
-
Ferricytochrome
References
Ansari A, Berendzen J, Bowne SF et al (1985) Protein states and proteinquakes. Proc Natl Acad Sci USA 82:5000–5004
Austin RH, Beeson KW, Eisenstein L (1975) Dynamics of ligand binding to myoglobin. Biochemistry 14:5355–5373
Baldwin RL (1986) Temperature dependence of the hydrophobic interaction in protein folding. Proc Natl Acad Sci USA 83:8069–8072
Benson SW, Dobis O (1998) Existence of negative activation energies in simple bimolecular metathesis reactions and some observations on too fast reactions. J Phys Chem A 102:5175–5181
Bhuyan AK (2002) Protein stabilization by urea and guanidine hydrochloride. Biochemistry 41:13386–13394
Bryngelson JD, Wolynes PG (1989) Intermdeiates and barrier crossing in a random energy model with applications to protein folding. J Phys Chem 93:6902–6915
Bryngelson JD, Onuchic JN, Socci ND, Wolynes PG (1995) Funnels, pathways and the energy landscape of protein folding: a synthesis. Proteins 21:167–195
Bushnell GW, Louie GV, Brayer GD (1990) High-resolution three-dimensional structure of horse heart cytochrome c. J Mol Biol 214:585–595
Campbell ID, Dobson CM, Moore GR, Perkins S.J, Williams RJP (1976) Temperature dependent molecular motion of a tyrosine residue of ferrocytochrome c. FEBS Lett 70:96–100
Chan B-l, Baase WA, Schellman JA (1989) Low-temperature unfolding of a mutant of phage T4 lysozyme. 2. Kinetic investigations. Biochemistry 28:691–699
Dill KA (1990) Dominant forces in protein folding. Biochemistry 29:7133–7155
Dill KA, Shortle D (1991) Denatured states of proteins. Annu Rev Biochem 60:795–825
Doan-Nguyen V, Loria JP (2007) The effects of cosolutes on protein dynamics: the reversal of denaturant-induced protein fluctuations by trimethylamine N-oxide. Protein Sci 16:20–29
Denisov VP, Peters J, Hörlein HD, Halle B (1996) Using buried water molecules to explore the energy landscape of proteins. Nat Struct Biol 3:505–509
Dunbar J, Yennawar HP, Banerjee S, Luo J et al (1997) The effects of denaturants on protein structure. Protein Sci 6:1272–1733
Elöve GA, Bhuyan AK, Roder H (1994) Kinetic mechanism of cytochrome c folding: involvement of the heme and its ligands. Biochemistry 33:6925–6935
Englander SW, Mayne L (1992) Protein folding studied using hydrogen-exchange labeling and two-dimensional NMR. Annu Rev Biophys Biomol Struct 21:243–265
Englander SW, Downer NW, Teitelbaum H (1972) Hydrogen exchange. Annu Rev Biochem 41:903–924
Ernst RR, Bodenhausen G, Wokaun A (1988) Principles of nuclear magnetic resonance in one and two dimensions. Clarendon Press, Oxford
Fejzo J, Zolnai Z, Macura S, Markley JL (1990) Quantitative evaluation of two-dimensional cross-relaxation NMR spectra of proteins. Interprotein distances in turkey ovomucoid third domain. J Magn Reson 88:93–110
Fejzo J, Westler WM, Macura S, Markley JL (1991) Strategies for eliminating unwanted cross-relaxation and coherence-transfer effects from two-dimensional chemical exchange spectra. J Magn Reson 92:20–29
Fenimore PW, Frauenfelder H, McMahon BH, Young RD (2004) Bulk-solvent and hydration-shell fluctuations, similar to alpha- and beta-fluctuations in glass, control protein motions and fluctuations. Proc Natl Acad Sci USA 101:14408–14413
Gall CM, Cross TA, DiVerdi JA, Opella SJ (1982) Protein dynamics by solid state NMR: aromatic rings of the coat protein in fd bateriophage. Proc Natl Acad Sci USA 79:101–105
Gallego J (2004) Sequence-dependent nucleotide dynamics revealed by intercalated ring rotation in DNA-bisnaphthalimide complexes. Nucleic Acid Res 32:3607–3614
Grishaev A, Wu J, Trewhella J, Bax A (2005) Refinement of multidomain protein structures by combination of solution small-angle X-ray scattering and NMR data. J Am Chem Soc 127:16621–16628
Hattori M, Li H, Yamada H, Akasaka K et al (2004) Infrequent cavity-forming fluctuations in HPr from Staphylococcus carnosus revealed by pressure- and temperature-dependent tyrosine ring flips. Protein Sci 13:3104–3114
Karplus M (1986) Internal dynamics of proteins. Methods Enzymol 131:283–307
Karplus M (2000) Aspects of protein reaction dynamics: deviations from simple behavior. J Phys Chem B 104:11–27
Kawahara K, Tanford C (1966) Viscosity and density of aqueous solutions of urea and guanidine hydrochloride. J Biol Chem 241:3228–3232
Khare R, Paulaitis ME (1995) A study of cooperative phenyl ring flip motions in glassy polystyrene by molecular simulations. Macromolecules 28:4495–4504
Krasnoperov LN, Peng J, Marshall P (2006) Modified transition state theory and negative apparent activation energies of simple metathesis reactions: application to the reaction CH3 + HBr → CH4 + Br. J Phys Chem A 110:3110–3120
Kumar R, Bhuyan AK (2005) Two-state folding of horse ferrocytochrome c: analyses of linear free energy relationship, chevron curvature, and stopped-flow burst relaxation kinetics. Biochemistry 44:3024–3033
Kumar R, Prabhu NP, Yadaiah M, Bhuyan AK (2004) Protein stiffening and entropic stabilization in the subdenaturing limit of guanidine hydrochloride. Biophys J 87:2656–2662
Lam PC-H, Carlier PR (2005) Experimental and computational studies of ring inversion of 1,4-benzodiazepin-2-ones: implications for memory of chirality transformations. J Org Chem 70:1530–1538
Li H, Yamada H, Akasaka K (1998) Effect of pressure on individual hydrogen bonds in proteins. Basic pancreatic trypsin inhibitor. Biochemistry 37:1167–1173
Maity H, Rumbley JN, Englander SW (2006) Functional role of a protein foldon- an omega-loop foldon controls the alkaline transition in ferricytochrome c. Proteins 63:349–355
Makhatadze GI, Privalov PL (1992) Protein interactions with urea and guanidinium chloride. A calorimetric study. J Mol Biol 226:491–505
McCammon JA, Wolynes PG, Karplus M (1979) Picosecond dynamics of tyrosine side chains in proteins. Biochemistry 18:927–942
Nall BT, Zuniga EH (1990) Rates and energetics of tyrosine ring flips in yeast iso-2-cytochrome c. Biochemistry 29:7576–7584
Okazaki K-i, Koga N, Takada S, Onuchic JN et al (2006) Multiple-basin energy landscapes for large-amplitude conformational motions of proteins: structure-based molecular dynamics simulations. Proc Natl Acad Sci USA 103:11844–11849
Oliveberg M, Tan Y-J, Fersht AR (1995) Negative activation enthalpies in the kinetics of protein folding. Proc Natl Acad Sci USA 92:8926–8929
Otting G, Liepinsh E, Wüthrich K (1993) Disulfide bond isomerization in BPTI and BPTI(G36S): an NMR study of correlated mobility in proteins. Biochemistry 32:3571–3582
Pellicena P, Kuriyan J (2006) Protein–protein interactions in the allosteric regulation of protein kinase. Curr Opin Struct Biol 16:702–709
Pike ACW, Acharya R (1994) A structural basis for the interaction of urea with lysozyme. Protein Sci 3:706–710
Portman JJ, Takada S, Wolynes PG (2001) Microscopic theory of protein folding rates. II. Local reaction coordinates and chain dynamics. J Chem Phys 114:5082–5096
Roder H (1989) Structural characterization of protein folding intermediates by proton magnetic resonance and hydrogen exchange. Methods Enzymol 176:446–473
Roder H, Elöve GA, Englander SW (1988) Structural characterization of folding intermediates in cytochrome c by H-exchange labeling and proton NMR. Nature 335:700–704
Scalley ML, Baker D (1997) Protein folding kinetics exhibit an Arrhenius temperature dependence when corrected for the temperature dependence of protein stability. Proc Natl Acad Sci USA 94:10636–10640
Shaw BF, Valentine JS (2007) How do ALS-associated mutations in superoxide dismutase 1 promote aggregation of the protein. Trends Biochem Sci 32:78–85
Shokhirev NV, Shokhireva TK, Polam JR, Watson CT et al (1997) 2D NMR investigations of the rotation of axial ligands in six-coordinate low-spin iron(III) and cobalt(III) tetraphenylporphyrinates having 2,6-disubstituted phenyl rings: quantitation of rate constants from 1H EXSY cross-peak intensities. J Phys Chem A 101:2778–2786
Skalicky JJ, Mills JL, Sharma S, Szyperski T (2001) Aromatic ring-flipping in supercooled water: implications for NMR-based structural biology of proteins. J Am Chem Soc 123:388–397
Stangler T, Hartmann R, Willbold D, Koenig BW (2006) Modern high resolution NMR for the study of structure, dynamics and interactions of biological macromolecules. Z Phys Chem 220:567–613
Steinbach PJ, Ansari A, Berendzen J et al (1991) Ligand binding to heme protein: connection between dynamics and function. Biochemistry 30:3988–4001
Truhlar DG, Kohen A (2001) Convex Arrhenius plots and their interpretation. Proc Natl Acad Sci USA 98:848–851
Wagner G, DeMarco A, Wüthrich K (1976) Dynamics of the aromatic amino acid residues in the globular conformation of the basic pancreatic trypsin inhibitor (BPTI). Biohys Struct Mechanism 2:139–158
Wagner G, Wüthrich K (1978) Dynamic model of globular protein conformations based on NMR studies in solution. Nature 275:247–248
Wallace MI, Ying L, Balasubramanian S, Klenerman D (2001) Non-Arrhenius kinetics for the loop closure of a DNA hairpin. Proc Natl Acad Sci USA 98:5584–5589
Whittaker SB-M, Boetzel R, MacDonald C, Lian L-Y et al (1998) NMR detection of slow conformational dynamics in an endonuclease toxin. J Biomol NMR 12:145–159
Wu LC, Laub PB, Elöve GA, Carey J et al (1993) A noncovalent peptide complex as a model for an early folding intermediate of cytochrome c. Biochemistry 32:10271–19276
Wüthrich K, Wagner G (1975) NMR investigations of the dynamics of the aromatic amino acid residues in the basic pancreatic trypsin inhibitor. FEBS Lett 50:265–268
Zarrine-Afsar A, Mittermaier A, Kay LE, Davidson AR (2006) Protein stabilization by specific binding of guanidinium to a functional arginine-binding surface on an SH3 domain. Protein Sci 15:162–170
Acknowledgments
This work was supported by grants from the Department of Biotechnology (BRB/15/227/2001), the Department of Science & Technology (4/1/2003-SF), and the University Grants Commission (UPE Funding), Government of India. AKB is the recipient of a Swarnajayanti Fellowship from the DST.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishna Rao, D., Bhuyan, A.K. Complexity of aromatic ring-flip motions in proteins: Y97 ring dynamics in cytochrome c observed by cross-relaxation suppressed exchange NMR spectroscopy. J Biomol NMR 39, 187–196 (2007). https://doi.org/10.1007/s10858-007-9186-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-007-9186-2