Abstract
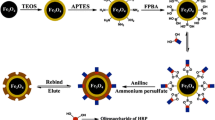
Protein imprinting is still a challenge due to the low binding kinetics and poor binding selectivity. In this study, a facile method of the preparation of magnetic molecularly imprinted polymers (MIPs) for selective protein separation was reported. Carboxyl group functionalized Fe\(_{3}\)O\(_{4}\) nanoparticles (NPs) were synthesized using a solvothermal method. After pre-assembly of caroxyl group functionalized Fe\(_{3}\)O\(_{4}\) NPs and template protein lysozyme (Lyz) to form Lyz–Fe\(_{3}\)O\(_{4}\) complex, magnetic MIPs were synthesized by a sol–gel process of 3-aminopropyltriethoxylsilane and tetraethyl silicate with Lyz–Fe\(_{3}\)O\(_{4}\) complex incorporated. Then Fe\(_{3}\)O\(_{4}\)–MIPs particles with magnetic response could be collected by simple crush of bulk polymers. This preparation process avoid the need of high dilution of monomer for anti-agglomeration in the surface imprinting, and large amount of solvent is spared. The morphology and structure property of the prepared magnetic NPs were characterized by transmission electronic microscopy, Fourier transform infrared spectroscopy, and vibrating sample magnetometer. Binding experiments were carried out to evaluate Fe\(_{3}\)O\(_{4}\)–MIPs particles’ binding performance and selectivity. And results showed fast binding kinetics, high binding capacity, and favorable specific recognition behavior toward template protein, which is due to the role of carboxyl group functionalized Fe\(_{3}\)O\(_{4}\) NPs as both magnetic source and importantly as co-functional monomer incorporated in the polysiloxane imprinting system. Real egg white sample tests demonstrate good separation effect. This report provides a possibility of the selective separation of protein in complex matrix.













Similar content being viewed by others
References
Tan CJ, Chua HG, Ker KH, Tong YW (2008) Preparation of bovine serum albumin surface-imprinted submicrometer particles with magnetic susceptibility through core-shell miniemulsion polymerization. Anal Chem 80:683–692
Alexander C, Andersson H, Andersson L, Ansell R, Kirsch N, Nicholls I, O’Mahony J, Whitcombe M (2006) Molecular imprinting science and technology: a survey of the literature for the years up to and including 2003. J Mol Recognit 19:106–180
Whitcombe MJ, Kirsch N, Nicholls IA (2014) Molecular imprinting science and technology: a survey of the literature for the years 2004–2011. J Mol Recognit 27:297–401
Shin MJ, Shin YJ, Hwang SW, Shin JS (2013) Recognizing amino acid chirality with surface-imprinted polymers prepared in W/O emulsions. Int J Polym Sci 2013:1–5
Gkementzoglou C, Kotrotsiou O, Kiparissides C (2013) Synthesis of novel composite membranes based on molecularly imprinted polymers for removal of triazine herbicides from water. Ind Eng Chem Res 52:14001–14010
Liu J, Yang M, Hou D, Hou C, Li X, Wang G, Feng D (2013) Molecularly imprinted polymers on the surface of silica microspheres via sol-gel method for the selective extraction of Streptomycin in aqueous samples. J Sep Sci 36:1142–1148
Xie X, Chen L, Pan X, Wang S (2015) Synthesis of magnetic molecularly imprinted polymers by reversible addition fragmentation chain transfer strategy and its application in the Sudan dyes residue analysis. J Chromatogr A 1405:32–39
Xie X, Pan X, Han S, Wang S (2015) Development and characterization of magnetic molecularly imprinted polymers for the selective enrichment of endocrine disrupting chemicals in water and milk samples. Anal Bioanal Chem 407:1735–1744
Xie X, Wei F, Chen L, Wang S (2015) Preparation of molecularly imprinted polymers based on magnetic nanoparticles for the selective extraction of protocatechuic acid from plant extracts. J Sep Sci 38:1046–1052
Miao S, Wu M, Zuo H, Jiang C, Jin S, Lu Y, Yang H (2015) Core-shell magnetic molecularly imprinted polymers as sorbent for sulfonylurea herbicide residues. J Agric Food Chem 63:3634–3645
Zhou Y, Zhou T, Jin H, Jing T, Song B, Zhou Y, Mei S, Lee Y (2015) Rapid and selective extraction of multiple macrolide antibiotics in foodstuff samples based on magnetic molecularly imprinted polymers. Talanta 137:1–10
Wang C, Hu X, Guan P, Qian L, Wu D, Li J (2015) Superparamagnetic molecularly imprinting polymers for adsorbent and separation pentapeptides by surface ATRP. Sep Sci Technol 50:1768–1775
Ma P, Zhou Z, Yang W, Tang B, Liu H, Xu W, Huang W (2015) Preparation and application of sulfadiazine surface molecularly imprinted polymers with temperature-responsive properties. J Appl Polym Sci 132:41769–41781
Chen H, Kong J, Yuan D, Fu G (2014) Synthesis of surface molecularly imprinted nanoparticles for recognition of lysozyme using a metal coordination monomer. Biosens Bioelectron 53:5–11
Gao R, Mu X, Hao Y, Zhang L, Zhang J, Tang Y (2014) Combination of surface imprinting and immobilized template techniques to preparation of core-shell molecularly imprinted polymers based on directly amino-modified Fe\(_{3}\)O\(_{4}\) nanoparticles for specific recognition of bovine hemoglobin. J Mater Chem B 2:1733–1741
Liu Y, Gu Y, Li M, Wei Y (2014) Protein imprinting over magnetic nanospheres via a surface grafted polymer for specific capture of hemoglobin. New J Chem 38:6064–6072
Li Q, Yang K, Liang Y, Jiang B, Liu J, Zhang L, Liang Z, Zhang Y (2014) Surface protein imprinted core-shell particles for high selective lysozyme recognition prepared by reversible addition-fragmentation chain transfer strategy. ACS Appl Mater Interfaces 6:21954–21960
Phan NV, Sussitz HF, Lieberzeit PA (2014) Polymerization parameters influencing the QCM response characteristics of BSA MIP. Biosensors 4:161–171
Wan W, Han Q, Zhang X, Xie Y, Sun J, Ding M (2015) Selective enrichment of proteins for MALDI-TOF MS analysis based on molecular imprinting. Chem Commun 51:3541–3544
Kameya M, Sakaguchi-Mikami A, Ferri S, Tsugawa W, Sode K (2015) Advancing the development of glycated protein biosensing technology next-generation sensing molecules. J Diabetes Sci Technol 9:183–191
Yang Y, He X, Wang Y, Li W, Zhang Y (2014) Epitope imprinted polymer coating CdTe quantum dots for specific recognition and direct fluorescent quantification of the target protein bovine serum albumin. Biosens Bioelectron 54:266–272
Yang K, Liu J, Li S, Li Q, Wu Q, Zhou Y, Zhao Q, Deng N, Liang Z, Zhang L, Zhang Y (2014) Epitope imprinted polyethersulfone beads by self-assembly for target protein capture from the plasma proteome. Chem Commun 50:9521–9524
Kryscio DR, Peppas NA (2012) Critical review and perspective of macromolecularly imprinted polymers. Acta Biomater 8:461–473
He H, Fu G, Wang Y, Chai Z, Jiang Y, Chen Z (2010) Imprinting of protein over silica nanoparticles via surface graft copolymerization using low monomer concentration. Biosens Bioelectron 26:760–765
Fu G, He H, Chai Z, Chen H, Kong J, Wang Y, Jiang Y (2011) Enhanced lysozyme imprinting over nanoparticles functionalized with carboxyl groups for noncovalent template sorption. Anal Chem 83:1431–1436
Zhang M, Zhang X, He X, Chen L, Zhang Y (2012) A self-assembled polydopamine film on the surface of magnetic nanoparticles for specific capture of protein. Nanoscale 4:3141–3147
Li X, Zhang B, Li W, Lei X, Fan X, Tian L, Zhang H, Zhang Q (2014) Preparation and characterization of bovine serum albumin surface-imprinted thermosensitive magnetic polymer microsphere and its application for protein recognition. Biosens Bioelectron 51:261–267
Gao R, Kong X, Wang X, He X, Chen L, Zhang Y (2011) Preparation and characterization of uniformly sized molecularly imprinted polymers functionalized with core-shell magnetic nanoparticles for the recognition and enrichment of protein. J Mater Chem 21:17863–17871
Li Y, Hong M, Bin Q, Lin Z, Cai Z, Chen G (2013) Novel composites of multifunctional Fe\(_{3}\)O\(_{4}\)@Au nanofibers for highly efficient glycoprotein imprinting. J Mater Chem B 1:1044–1051
Herdt AR, Kim BS, Taton TA (2007) Encapsulated magnetic nanoparticles as supports for proteins and recyclable biocatalysts. Bioconj Chem 18:183–189
Gai Q, Qu F, Liu Z, Dai R, Zhang Y (2010) Superparamagnetic lysozyme surface-imprinted polymer prepared by atom transfer radical polymerization and its application for protein separation. J Chromatogr A 1217:5035–5042
Hua Z, Chen Z, Li Y, Zhao M (2008) Thermosensitive and salt-sensitive molecularly imprinted hydrogel for bovine serum albumin. Langmuir 24:5773–5780
Ou S, Wu M, Chou T, Liu C (2004) Polyacrylamide gels with electrostatic functional groups for the molecular imprinting of lysozyme. Anal Chim Acta 504:163–166
Odabaşi Say MR, Denizli A (2007) Molecular imprinted particles for lysozyme purification. Mater Sci Eng C 27:90–99
Hawkins DM, Stevenson D, Reddy SM (2005) Investigation of protein imprinting in hydrogel-based molecularly imprinted polymers (HydroMIPs). Anal Chim Acta 542:61–65
Ghasemzadeh N, Nyberg F, Hjertén S (2008) Highly selective artificial gel antibodies for detection and quantification of biomarkers in clinical samples. I. Spectrophotometric approach to design the calibration curve for the quantification. J Sep Sci 31:3945–3953
Ghasemzadeh N, Nyberg F, Hjertén S (2008) Highly selective artificial gel antibodies for detection and quantification of biomarkers in clinical samples. II. Albumin in body fluids of patients with neurological disorders. J Sep Sci 31:3954–3958
Chen Z, Hua Z, Xu L, Huang Y, Zhao M, Li Y (2008) Protein-responsive imprinted polymers with specific shrinking and rebinding. J Mol Recognit 21:71–77
Lina HY, Hoa MS, Lee MH (2009) Instant formation of molecularly imprinted poly(ethylene-co-vinyl alcohol)/quantum dot composite nanoparticles and their use in one-pot urinalysis. Biosens Bioelectron 25:579–586
Lee M, Chen Y, Ho M, Lin H (2010) Optical recognition of salivary proteins by use of molecularly imprinted poly (ethylene-co-vinyl alcohol)/quantum dot composite nanoparticles. Anal Bioanal Chem 397:1457–1466
Saridakis E, Khurshid S, Govada L, Phan Q, Hawkins D, Crichlow GV, Lolis E, Reddy SM, Chayen NE (2011) Protein crystallization facilitated by molecularly imprinted polymers. Proc Natl Acad Sci 108:11081–11086
Brüggemann O, Haupt K, Ye L, Yilmaz E, Mosbach K (2000) New configurations and applications of molecularly imprinted polymers. J Chromatogr A 889:15–24
Sulitzky C, Rückert B, Andrew J, Lanza F, Unger K, Sellergren B (2002) Grafting of molecularly imprinted polymer films on silica supports containing surface-bound free radical initiators. Macromolecules 35:79–91
Kempe H, Kempe M (2004) Novel method for the synthesis of molecularly imprinted polymer bead libraries. Macromol Rapid Commun 25:315–320
Liu J, Sun Z, Deng Y, Zou Y, Li C, Guo X, Xiong L, Gao Y, Li F, Zhao D (2009) Highly water-dispersible biocompatible magnetite particles with low cytotoxicity stabilized by citrate groups. Angew Chem 121:5989–5993
Jing T, Du H, Dai Q, Xia H, Niu J, Hao Q, Mei S, Zhou Y (2010) Magnetic molecularly imprinted nanoparticles for recognition of lysozyme. Biosens Bioelectron 26:301–306
Cao J, Zhang X, He X, Chen L, Zhang Y (2014) The synthesis of magnetic lysozyme-imprinted polymers by means of distillation-precipitation polymerization for selective protein enrichment. Chem Asian J 9:526–533
Zhang M, Wang Y, Jia X, He M, Xu M, Yang S, Zhang C (2014) The preparation of magnetic molecularly imprinted nanoparticles for the recognition of bovine hemoglobin. Talanta 120:376–385
Kan X, Zhao Q, Shao D, Geng Z, Wang Z, Zhu J (2010) Preparation and recognition properties of bovine hemoglobin magnetic molecularly imprinted polymers. J Phys Chem B 114:3999–4004
Liu Y, Liu Z, Gao J, Dai J, Han J, Wang Y, Xie J, Yan Y (2011) Selective adsorption behavior of Pb (II) by mesoporous silica SBA-15-supported Pb (II)-imprinted polymer based on surface molecularly imprinting technique. J Hazard Mater 186:197–205
Umpleby R, Baxter S, Chen Y, Shah R, Shimizu K (2001) Characterization of molecularly imprinted polymers with the Langmuir-Freundlich isotherm. Anal Chem 73:4584–4591
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, S., Zhang, X., Zhao, W. et al. Preparation and evaluation of Fe\(_{3}\)O\(_{4}\) nanoparticles incorporated molecularly imprinted polymers for protein separation. J Mater Sci 51, 937–949 (2016). https://doi.org/10.1007/s10853-015-9423-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9423-0