Abstract
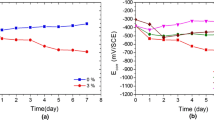
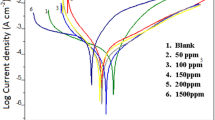
The influence of sodium nitrite on the corrosion processes of iron in solutions simulating polluted concrete was investigated by means of electrochemical methods, such us potentiodynamic, galvanostatic and impedance spectroscopy tests, coupled with analyses of the corrosion layers by Mössbauer spectroscopy. NO −2 ions are anodic inhibitors and provoked consequently an important increase of the corrosion potential. The polarisation curves show that NO −2 ions increase the pitting potential value. The size of electrochemical impedance spectra obtained at the OCP increases with the concentration of nitrite, which confirms the decrease of the corrosion rate. Galvanostatic experiments allowed us to provoke active corrosion even in presence of NO −2 . When nitrite ions are not present, the corrosion products mainly consist of iron (II) compounds, FeCO3 or Fe(OH)2 depending on the pH, and iron(II)–iron (III) compounds, i.e. green rusts (GRs). The main effect of nitrite ions was to accelerate the oxidation of GRs into FeOOH phases, confirming their oxidizing role. While immersed for long periods in the nitrite containing solutions, the α-iron foils do not present any single trace of corrosion.








Similar content being viewed by others
References
Refait Ph, Génin J-MR (1993) Corros Sci 33:797
Drissi SH, Refait Ph, Abdelmoula M, Génin J-MR (1995) Corros Sci 37:2025
Abdelmoula M, Refait Ph, Drissi SH, Mihé J-P, Génin J-MR (1996) Corros Sci 38:623
Refait Ph, Abdelmoula M, Génin J-MR (1998) Corros Sci 40:1547
Dhouibi L, Refait Ph, Abdelmoula M, Triki E, Génin J-MR (2002) Corrosion 58:467
Hachani L, Carpio J, Fiaud C, Raharinaivo A, Triki E (1992) Cement Concrete Res 22:56
Pruckner F (2001) Thesis, Faculty Nat Sci Math. Vienna
Detournay J, Dérie R, Ghodsi M (1976) Z anorg allg Chem 427:265
Schwertmann U, Fechter H (1994) Clay Miner 29:87
Simon L, Génin J-MR, Refait Ph (1997) Corros Sci 39:1673
Mendiboure A, Schöllhorn R (1986) Rev Chim Min 23:819
Refait Ph, Drissi SH, Pytkiewicz J, Génin JM (1997) Corros Sci 39:1699
Forester DW, Koon NC (1969) J Appl Phys 40:1316
Benali O, Abdelmoula M, Refait Ph, Génin J-MR (2001) Geochim et Cosmochim Acta 65:1715
Rossiter MJ, Hodgson AEM (1965) J Inorg Nucl Chem 27:63
Morup S, Madsen MB, Franck J, Villadsen J, Koch CJW (1983) J Magn Magn Mater 40:163
Murad E, Schwertmann U (1980) Amer Min 65:1044
Refait Ph, Génin J-MR (1997) Corros Sci 39:539
Stampfl PP (1969) Corros Sci 9:185
Pryor MJ, Cohen M (1953) J Electrochem Soc 100:203
Mayne JEO, Menter JW (1954) J Chem Soc 103
Szklarska-Smialowska Z, Staehle RW, (1974) J Electrochem Soc 121:1393
Berke N, Hicks MC (1997) Conf. on understanding corrosion mechanisms in concrete, Cambridge, Massachusetts, USA
Hansen HCB, Borggaard OK, Sorensen J (1994) Geochim Cosmochim Acta 58:2599
Acknowledgements
The authors would like to thank Dr. Mustapha Abdelmoula for his help in accomplishing the Mössbauer experiments. This study was made possible by the DGRST-CNRS agreement between Tunisia and France and a grant to one of them (L. D.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhouibi, L., Refait, P., Triki, E. et al. Interactions between nitrites and Fe(II)-containing phases during corrosion of iron in concrete-simulating electrolytes. J Mater Sci 41, 4928–4936 (2006). https://doi.org/10.1007/s10853-006-0332-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-0332-0