Abstract
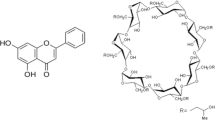
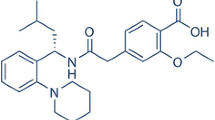
The objectives of this research were to prepare and characterize inclusion complex of Ezetimibe (EZE) with cyclodextrins (β-cyclodextrin (β-CD) and hydroxypropyl-β-cyclodextrin (HPβ-CD)) and to study the effect of complexation on the dissolution rate of EZE, a water insoluble drug. Phase solubility curve was classified as A P -type for both cyclodextrins, indicating the 2:1 stoichiometric ratio for β-CD–EZE and HPβ-CD – EZE inclusion complexes. The inclusion complexes in the molar ratio of 2:1 (β-CD–EZE and HPβ-CD–EZE) were prepared by various methods such as kneading, coevaporation and physical mixing. The molecular behaviors of drug in all samples were characterized by fourier-transform infrared (FTIR) spectroscopy, differential scanning calorimetry (DSC) and powder X-ray diffraction (PXRD) studies. The results of these studies indicated that complex prepared by kneading and coevaporation methods showed inclusion of the EZE molecule into the cyclodextrins cavities. The highest improvement in in-vitro dissolution profiles was observed in complex prepared with hydroxypropyl-β-cyclodextrin using co-evaporation method. Mean dissolution time and similarity factor indicated significant difference between the release profiles of EZE from complexes and physical mixtures and from pure EZE.







Similar content being viewed by others
References
Proudfoot, S.: Factors affecting bioavailability: factors influencing drug absorption from the gastrointestinal tract. In: Aulton, M.E. (Ed.), Pharmaceutics: The Science of Dosage From Design, pp. 135–173. Churchill Livingstone London, UK (1991)
Pitha, J., Milecki. J., Fales, H., Pannell, L., Uekama, K.: Hydroxypropyl-β-cyclodextrin: preparation and characterization; Effects on solubility of drugs. Int. J. Pharm. 29: 73–82 (1986)
Duchêne, D.: Cyclodextrins and their industrial uses. Editions de Santé Paris, SS. 447–460 (1987)
Uekama, K., Otagiri, M.: Cyclodextrins in drug carrier systems. Crit. Rev. Ther. Drug Carrier Syst. 3, 1–40 (1987)
Szejtli, J.: Medicinal applications of cyclodextrins. Med. Res. Rev. 14, 353–386 (1994)
Baboota, S., Dhaliwal, M., Kohli, K.: Physicochemical characterization, in vitro dissolution behavior, and pharmacodynamic studies of rofecoxib-cyclodextrin inclusion compounds. AAPS Pharm. Sci. Tech. 6, E83–E90 (2005)
Ghorab, M.K., Adeyeye, M.C.: Elucidation of solution state complexation in wet-granulated oven-dried ibuprofen and β-cyclodextrin: FT–IR and 1H–NMR studies. Pharm. Dev. Technol., 6, 315–324 (2001)
Veiga, F.J., Fernandes, C., Carvalho, R.A., Geraldes, C.F.: Molecular modeling and 1H–NMR: ultimate tools for the investigation of tolbutamide: β-cyclodextrin and tolbutamide: hydroxypropyl-β-cyclodextrin complexes. Chem. Pharm. Bull. 49, 1251–1256 (2001)
Tirucherai, G.S., Mitra, A.K.: Effect of hydroxypropyl beta cyclodextrin complexation on aqueous solubility, stability, and corneal permeation of acyl ester prodrugs of ganciclovir. AAPS Pharm. Sci. Tech. 4, E45 (2003)
Nalluri, B.N., Chowdary, K.P.R., Murthy, K.V.R., Hayman, A.R., Becket, G.: Physicochemical characterization and dissolution properties of nimesulide-cyclodextrin binary systems. AAPS Pharm. Sci. Tech. 4, E2 (2003)
Moore, J.W., Flanner, H.: Mathematical comparison of dissolution profiles. Pharm. Tech. 20, 64–74 (1996)
Reppas, C., Nicolaides, E.: Analysis of drug dissolution data, In: Dressman, J.B., Lennernäs, H. (Eds.), Oral drug absorption prediction and assessment, pp. 229–254. Marcel Dekker, New York (2000)
Vueba, M.L., Batista de Carvalho, L.A.E., Veiga, F., Sousa, J.J., Pina, M.E.: Influence of cellulose ether polymers on ketoprofen release from hydrophilic matrix tablets. Eur. J. Pharm. Biopharm. 58, 51–59 (2004)
Higuchi, T., Connors, K.: Phase solubility techniques. Adv. Anal. Chem. Instru. 4, 17–123 (1965)
Peri, D., Wyandt, C.M., Cleary, R.W., Hickal, A.H., Jones, A.B.: Inclusion complexes of tolnaftate with β- Cyclodextrin and hydroxy-β-cyclodextrin. Drug Dev. Ind. Pharm. 20, 1401–1410 (1994)
Labenderia, J.J.T., Lopez, M.E., Penin, L.S., Jato, J.L.V.: Glibornuride-β-cyclodextrin inclusion complexes: Preparation, structural characterization, and in vitro dissolution behavior. J. Pharm. Biopharm., 39, 255–259 (1993)
Chengsheng, L., Kashappa, H.D.: Enhancement of dissolution rate of valdecoxib using solid dispersions with polyethylene glycol 4000. Drug Dev. Ind. Pharm. 1, 1–10 (2005)
Hedges, A.R.: Industrial applications of cyclodextrins. Chem. Rev. 98, 2035– 2044 (1998)
Vromans, H., Eisson, A.C., Coenraad, F.L.: Mechanism of dissolution of drug-cyclodextrin complexes. Drug Dev. Ind. Pharm. 15, 250–255 (1989)
Acknowledgements
We would like to thank Zydus Cadila, India for donating EZE and conducting PXRD studies of the samples. We are grateful to Maan Pharmaceuticals Ltd. for providing formulation excipients.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, R., Bhimani, D., Patel, J. et al. Solid-state characterization and dissolution properties of ezetimibe–cyclodextrins inclusion complexes. J Incl Phenom Macrocycl Chem 60, 241–251 (2008). https://doi.org/10.1007/s10847-007-9371-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-007-9371-7