Abstract
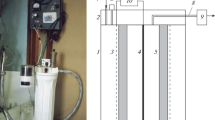
The results of an experimental study on electrochemical disinfection of water are presented. Attention was paid to the behaviour of chlorine compounds during electrolysis of water containing chlorides, with particular regard to the selectivity of the process towards the production of oxidising agents. Two reactor configurations were tested: a stirred tank cell and a filter press cell inserted in a hydraulic circuit. Both cells were equipped with boron doped diamond (BDD) anodes. Experiments were performed in batch and continuous mode. The effect of such operating parameters, current density, stirring rate or recirculating flow rate, on the behaviour of the process was investigated. The results at BDD anodes show that low current densities and perfect mixing of the system should be adopted in order to obtain high values of the concentration of oxidising agents avoiding the formation of such undesired by-products as chlorite, chlorate and perchlorate ions. Runs were also performed in which BDD was substituted by a commercial (Ti/RuO2) DSA anode and the results obtained with the two materials are compared.








Similar content being viewed by others
References
Rychen P, Pupunat L, Haenni W, Santoli E (2003) New Diamond Front Carbon Technol 13:109–117
Foti G, Gandini D, Comninellis C, Perret A, Haenni W (1999) Electrochem Solid State Lett 2:228–230
Santana MHP, Faria LAD, Boodts JFC (2005) Electrochim Acta 50:2017–2027
Serrano K, Michaud PA, Comninellis C, Savall A (2002) Electrochim Acta 48:431–436
Pacheco MJ, Morao A, Lopes A, Ciriaco L, Goncalves I (2007) Electrochim Acta 53:629–636
Zhu X, Shi S, Wei J, Lv F, Zhao H, Kong J, He Q, Ni J (2007) Environ Sci Technol 41:6541–6546
Yavuz Y, Koparal AS, Ogutveren UB (2008) J Environ Eng 134:24–31
Flox C, Cabot PL, Centellas F, Garrido JA, Rodrıguez RM, Arias C, Brillas E (2007) Appl Catal B Environ 75:17–28
Saez C, Panizza M, Rodrigo MA, Cerisola G (2007) J Chem Technol Biotechnol 82:575–581
Polcaro AM, Vacca A, Mascia M, Palmas S (2005) Electrochim Acta 50:1841–1847
Alfaro MAQ, Ferro S, Martinez-Huitle CA, Vong YM (2006) J Brazil Chem Soc 17:227–236
Mascia M, Vacca A, Palmas S, Polcaro AM (2007) J Appl Electrochem 37:71–76
Polcaro AM, Vacca A, Mascia M, Palmas S, Pompei R, Laconi S (2007) Electrochim Acta 52:2595–2602
Jeong J, Kim JY, Yoon J (2006) Environ Sci Technol 40:6117–6122
Bergmann MEH, Rollin J (2007) Catal Today 124:198–203
Guidelines for Drinking-water Quality Volume 1 (2006) World Health Organization, Geneva
Bergmann MEH, Koparal AS (2005) Electrochim Acta 50:5218–5228
Fierro S, Nagel T, Baltruschat H, Comninellis C (2007) Electrochem Commun 9:1969–1974
Trasatti S (1994) Electrochemistry of Novel Materials. VCH Publishers, New York, p 238
Bergmann MEH, Koparal AS (2005) J Appl Electrochem 35:1321–1329
Marselli B, Garcia-Gomez J, Michaud PA, Rodrigo MA, Comninellis C (2003) J Electrochem Soc 150:D79–D83
Acknowledgment
The authors gratefully acknowledge the University of Cagliari for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Polcaro, A.M., Vacca, A., Mascia, M. et al. Product and by-product formation in electrolysis of dilute chloride solutions. J Appl Electrochem 38, 979–984 (2008). https://doi.org/10.1007/s10800-008-9509-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-008-9509-3