Abstract
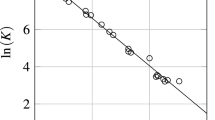
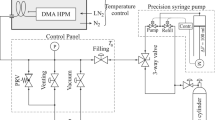
Vapor pressures of the 1,1,1,2-tetrafluoroethane + polyalkylene glycol system were obtained at 72 points over the temperature range from 253.15 to 333.15 K at 10 K intervals and the composition range from 0 to 90 mass % polyalkylene glycol. It was found that below 273.15 K, the effect of the polyalkylene glycol on the vapor pressure was negligible up to 30 mass % polyalkylene glycol. The vapor pressure of the 1,1,1,2-tetrafluoroethane + polyalkylene glycol system decreased as the concentration of polyalkylene glycol increased. Raoult’s model and Flory–Huggins model were used for data reduction. Raoult’s model gave reasonable predictions for the vapor pressure of the system below 30 mass % polyalkylene glycol. The Flory–Huggins model gave reasonable predictions for the vapor pressure over the complete composition range. An empirical vapor pressure equation was obtained in terms of temperature and mass fraction polyalkylene glycol. The empirical equation was the most convenient way to calculate the vapor pressure.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, Y.M., Kang, J.O., Yoo, J. et al. Vapor Pressure of the 1,1,1,2-Tetrafluoroethane (R-134a) + Polyalkylene Glycol System. Int J Thermophys 25, 1849–1861 (2004). https://doi.org/10.1007/s10765-004-7739-0
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10765-004-7739-0