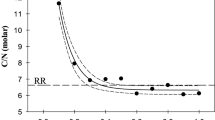
Abstract
Heterocytous Cyanobacteria show high trait variation at the cellular, organismal, and population levels. Members of this group can produce specialized cells such as akinetes and heterocytes that influence their ecology, including bloom development and population survival. This study characterizes patterns of variation in the traits of these species, including the traits of specialized cells, to expand our ecological knowledge and predictive capacity for this group. We compiled and synthesized morphological and physiological traits of planktic heterocytous Cyanobacteria from the published literature and experiments, and assessed trait distributions, trait relationships, and their similarities among species. Although the volumes of akinetes and heterocytes were positively related to that of vegetative cells, the shape of cells differed in ways that may reflect their function, and the position of heterocytes within filaments may relate to growth rate. Maximum growth rates differed significantly among genera, yet surprisingly did not correlate with cell volume. Also, despite the high energetic cost of N fixation in low N conditions, our results suggest that growth rate seems unrelated to nitrogen availability. The degree of trait variation within heterocytous Cyanobacteria, which suggests the existence of three functionally distinct subgroups, may offer new insights into which taxa dominate bloom assemblages under different conditions.





Similar content being viewed by others
References
Adams, D. G. & P. S. Duggan, 1999. Heterocyst and akinete differentiation in cyanobacteria. New Phytologist 144: 3–33.
Bates, D., M. Mächler, B. Bolker & S. Walker, 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48.
Braune, W., 1980. Structural aspects of akinete germination in the cyanobacterium Anabaena variabilis. Archives of Microbiology 126: 257–261.
Bruggeman, J., 2011. A phylogenetic approach to the estimation of phytoplankton traits. Journal of Phycology 47: 52–65.
Burford, M. A., J. Beardall, A. Willis, P. T. Orr, V. T. Magalhaes, L. M. Rangel, S. M. O. F. Azevedo & B. A. Neilan, 2016. Understanding the winning strategies used by the bloom-forming cyanobacterium Cylindrospermopsis raciborskii. Harmful Algae 54: 44–53.
de Tezanos Pinto, P., A. Kust, M. Devercelli & E. Kozlíková-Zapomělová, 2016. Morphological traits in nitrogen fixing heterocytous cyanobacteria: possible links between morphology and eco-physiology. Hydrobiologia 764: 271–281.
Dolman, A. M., J. Rücker, F. R. Pick, J. Fastner, T. Rohrlack, U. Mischke & C. Wiedner, 2012. Cyanobacteria and cyanotoxins: the influence of nitrogen versus phosphorus. PLoS ONE 7: e38757.
Fogg, G. E. & B. Thake, 1987. Algal cultures and phytoplankton ecology. University of Wisconsin Press, Madison.
Halekoh, U. & S. Højsgaard, 2014. A Kenward–Roger approximation and parametric bootstrap methods for tests in linear mixed models – The R Package pbkrtest. Journal of Statistical Software 59: 1–32.
Hense, I. & A. Beckmann, 2006. Towards a model of cyanobacteria life cycle-effects of growing and resting stages on bloom formation of N2-fixing species. Ecological Modelling 195: 205–218.
Hillebrand, H., C.-D. Dürselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424.
Hindák, F., 1999. Akinete development in Anabaena augstumalis Schmidle (Cyanophyta/Cyanobacteria) by fusion of several pro-akinetes. Algological Studies 94: 147–161.
Hindák, F., 2008. Colour Atlas of Cyanophytes. Academy of Sciences, Veda Bratislava.
Huang, B., D. A. T. Harper & Ø. Hammer, 2013. Introduction to PAST, a comprehensive statistics software package for paleontological data analysis. Acta Palaeontologica Sinica 52: 161–181.
Kenesi, G., H. M. Shafik, A. W. Kovács, S. Herodek & M. Présing, 2009. Effect of nitrogen forms on growth, cell composition and N2 fixation of Cylindrospermopsis raciborskii in phosphorus-limited chemostat cultures. Hydrobiologia 623: 191–202.
Kerkhoff, A. J. & B. J. Enquist, 2009. Multiplicative by nature: why logarithmic transformation is necessary in allometry. Journal of Theoretical Biology 257: 519–521.
Kirk, J. T. O., 1994. Light and photosynthesis in aquatic ecosystems, 2nd ed. Cambridge University Press, Cambridge.
Kokociński, M. & J. Soininen, 2012. Environmental factors related to the occurence of Cylindrospermopsis raciborskii (Nostocales, Cyanophyta) at the north-eastern limit of its geographical range. European Journal of Phycology 47: 12–21.
Komárek, J., 2013. Cyanoprokaryota 3 Teil/3rd Part: heterocytous genera. In Büdel, B., G. Gaärtner, L. Krienitz & M. Schagerl (eds), Süwasserflora von mitteleuropa/freshwater flora of Central Europe 19/3. Berlin, Heidelberg, Springer Spektrum.
Kremer, C. T., M. K. Thomas & E. Litchman, 2017a. Temperature- and size-scaling of phytoplankton population growth rates: reconciling the Eppley curve and the metabolic theory of ecology. Limnology and Oceanography 62: 1658–1670.
Kremer, C. T., A. K. Williams, M. Finiguerra, A. A. Fong, A. Kellerman, S. F. Paver, B. B. Tolar & B. J. Toscano, 2017b. Realizing the potential of trait-based aquatic ecology: new tools and collaborative approaches. Limnology and Oceanography 62: 253–271.
Kruk, C., V. L. M. Huszar, E. T. H. M. Peeters, S. Bonilla, L. Costa, M. LüRling, C. S. Reynolds & M. Scheffer, 2010. A morphological classification capturing functional variation in phytoplankton. Freshwater Biology 55: 614–627.
Kruk, C., M. Devercelli, V. L. M. Huszar, E. Hernández, G. Beamud, M. Diaz, L. H. S. Silva & A. M. Segura, 2017. Classification of Reynolds phytoplankton functional groups using individual traits and machine learning techniques. Freshwater Biology 62: 1681–1692.
Kumar, K., R. A. Mella-Herrera & J. W. Golden, 2010. Cyanobacterial heterocysts. Cold Spring Harbor Perspective in Biology. https://doi.org/10.1101/cshperspect.a000315.
Lang, N. J. & P. Fay, 1971. The heterocysts of blue-green algae. II. Details of ultrastructure. Proceedings of the Royal Society B: Biological Sciences 178: 193–203.
Legrand, B., A. H. Le Jeune, J. Colombet, A. Thouvenot & D. Latour, 2017. Akinetes may be representative of past Nostocalean blooms: a case study of their benthic spatiotemporal distribution and potential for germination in a Eutrophic lake. Applied and Environmental Microbiology. https://doi.org/10.1128/AEM.01571-17.
Litchman, E. & C. A. Klausmeier, 2008. Trait-based community ecology of phytoplankton. Annual Review of Ecology, Evolution, and Systematics 39: 615–639.
Mantzouki, E., P. M. Visser, M. Bormans & B. W. Ibelings, 2016. Understanding the key ecological traits of cyanobacteria as a basis for their management and control in changing lakes. Aquatic Ecology Springer, Netherlands 50: 333–350.
Moore, D., G. B. McGregor & G. Shaw, 2004. Morphological changes during akinete germination in Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria). Journal of phycology 40: 1098–1105.
Nichols, J. M. & D. G. Adams, 1982. Akinetes. In Carr, N. G. & B. A. Whitton (eds), The Biology of Cyanobacteria. Blackwell, Oxford: 387–412.
O’Farrell, I., P. de Tezanos Pinto & I. Izaguirre, 2007. Phytoplankton morphological response to the underwater light conditions in a vegetated wetland. Hydrobiologia 578: 65–77.
O’Farrell, I., A. Vinocur & P. de Tezanos Pinto, 2015. Long-term study of bloom-forming cyanobacteria in a highly fluctuating vegetated floodplain lake: a morpho-functional approach. Hydrobiologia 752: 91–102.
Padisák, J., L. O. Crossetti & L. Naselli-Flores, 2009. Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621: 1–19.
Paerl, H. W. & J. Huisman, 2008. Blooms like it hot. Science American Association for the Advancement of Science 320: 57–58.
Pinheiro, J., D. Bates, S. DebRoy, D. Sarkar & R Core Team, 2018. nlme: linear and nonlinear mixed effects models. R package version 3.1-131.1. https://CRAN.R-project.org/package=nlme.
R Development Core Team, 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Reynolds, C. S., 1997. Vegetation Processes in the Pelagic: A Model for Ecosystem Theory, Vol. 9. Ecology Institute, Oldendorf/Luhe.
Reynolds, C. S., 2006. Ecology of Phytoplankton. Cambridge University Press, New York.
Reynolds, C. S., V. Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24: 417–428.
Sarthou Suárez, F. V., 2016. Floraciones de cianobacterias: efectos de la eutrofización y la variabilidad climática. Universidad de la República, Uruguay.
Schwaderer, A. S., K. Yoshiyama, P. de Tezanos Pinto, N. G. Swenson, C. A. Klausmeier & E. Litchman, 2011. Eco-evolutionary differences in light utilization traits and distributions of freshwater phytoplankton. Limnology and Oceanography 56: 589–598.
Sukenik, A., R. N. Kaplan-Levy, J. M. Welch & A. F. Post, 2011. Massive multiplication of genome and ribosomes in dormant cells (akinetes) of Aphanizomenon ovalisporum (Cyanobacteria). The ISME Journal Nature Publishing Group 6: 670–679.
Sukenik, A., R. N. Kaplan-Levy, Y. Viner-Mozzini, A. Quesada & O. Hadas, 2013. Potassium deficiency triggers the development of dormant cells (akinetes) in Aphanizomenon ovalisporum (Nostocales, Cyanoprokaryota) 1. Journal of Phycology 49: 580–587.
Tomitani, A., A. H. Knoll, C. M. Cavanaugh & T. Ohno, 2006. The evolutionary diversification of cyanobacteria: molecular-phylogenetic and paleontological perspectives. Proceedings of the National Academy of Sciences of the United States of America 103: 5442–5447.
Uyeda, J. C., L. J. Harmon & C. E. Blank, 2016. A comprehensive study of cyanobacterial morphological and ecological evolutionary dynamics through deep geologic time. PLoS ONE 11: 1–32.
Walsby, A. E., 2007. Cyanobacterial heterocysts: terminal pores proposed as sites of gas exchange. Trends in Microbiology 15: 340–349.
Wolk, C. P., A. Ernst & J. Elhai, 1994. Heterocyst metabolism and development. In Bryant, D. A. (ed.), The Molecular Biology of Cyanobacteria. Springer, Dordrecht: 769–823.
Yema, L., E. Litchman & P. de Tezanos Pinto, 2016. The role of heterocytes in the physiology and ecology of bloom-forming harmful cyanobacteria. Harmful Algae 60: 131–138.
Zevenboom, W., J. van der Does, K. Bruning & L. R. Mur, 1981. A non-heterocystous mutant of Aphanizomenon flosaquae, selected by competition in light-limited continuous culture. FEMS Microbiology Letters 10: 11–16.
Zhang, C.-C., S. Laurent, S. Sakr, L. Peng & S. Bédu, 2006. Heterocyst differentiation and pattern formation in cyanobacteria: a chorus of signals. Molecular Microbiology 59: 367–375.
Acknowledgements
We are grateful to researchers Ruben Lombardo, Martín Graziano, and Diego Frau for extending the statistics assistance. PTP acknowledges the funding from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina, PIP 1142010100236.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Judit Padisák
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yema, L., Kremer, C.T., O’Farrell, I. et al. Assessing patterns of morphological and physiological trait variations across heterocytous cyanobacteria at cellular and population levels. Hydrobiologia 823, 93–107 (2018). https://doi.org/10.1007/s10750-018-3698-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3698-5