Abstract
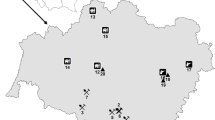
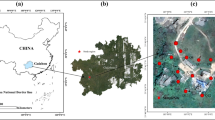
An investigation of the distribution, fractionation and phytoavailability of antimony (Sb) and other heavy metals in soil sampled at various locations in the vicinity of a Sb mine revealed elevated levels of Sb, most certainly due to the mining activities. The concentration of Sb in the soil samples was 100.6–5045 mg kg−1; in comparison, the maximum permissible concentration for Sb in soil in The Netherlands is 3.5 mg kg−1, and the maximum permissible concentration of pollutant Sb in receiving soils recommended by the World Health Organization is 36 mg kg−1. The soil sampled near the Sb mine areas had also contained high concentrations of As and Hg. Root and leaf samples from plants growing in the Sb mine area contained high concentrations of Sb, with the concentration of Sb in the leaves of radish positively correlating with Sb concentrations in soil. The distribution of Sb in the soil showed the following order: strongly bound to the crystalline matrix > adsorbed on Fe/Mn hydrous oxides, complexed to organic/sulfides, bound to carbonates > weakly bound and soluble. Solvents showed varying levels of effectiveness in extracting Sb (based on concentration) from the soil, with \({\hbox{Sb}}_{{\hbox{NH}}_{\hbox{4}} {\hbox{NO}}_{\hbox{3}} } {\hbox{ $ > $ Sb}}_{{\hbox{EDTA}}} {\hbox{ $ > $ Sb}}_{{\hbox{HAc}}} {\hbox{, Sb}}_{{\hbox{H}}_{\hbox{2}} {\hbox{O}}} {\hbox{ $ > $ Sb}}_{{\hbox{NH}}_{\hbox{4}} {\hbox{OAc}}}\), in decreasing order. The concentration of easily phytoavailable Sb was high and varied from 2.5 to 13.2 mg kg−1, the percentage of moderately phytoavailable Sb ranged from 1.62 to 8.26%, and the not phytoavailable fraction represented 88.2–97.9% of total Sb in soils.



Similar content being viewed by others
References
Afnor. (1994). Qualite des sols. Methodes d’analyses – recueil de norms francaises. Paris: Afnor.
Ainsworth, N., Cooke, J.A., & Johnson, M.S. (1990a). Distribution of antimony in contaminated grassland: I-Vegetation and soils. Environmental Pollution, 65, 65–77.
Ainsworth, N., Cooke, J.A., & Johnson, M.S. (1990b). Distribution of antimony in contaminated grassland: II-Small mammals and invertebrates. Environmental Pollution, 65, 79–87.
Ainsworth, N., Cooke, J. A., & Johnson, M. S. (1991). Biological significance of antimony in contaminated grassland. Water, Air and Soil Pollution, 57–58, 193–199.
Ashley, P. M., Craw, D., Graham, B. P., & Chappell, D. A. (2003). Environmental mobility of antimony around mesothermal stibnite deposits, New South Wales, Australia and southern New Zealand. Journal of Geochemical Exploration, 77, 1–14.
Baroni, F., Boscagli, A., Protano, G., & Riccobono, F. (2000). Antimony accumulation in Achillea ageratum, Plantago lanceolata and Silene vulgaris growing in an old Sb-mining area. Environmental Pollution, 109, 347–352.
Belzile, N., Chen, Y. W., & Wang, Z. J. (2001). Oxidation of antimony (III) by amorphous iron and manganese oxyhydroxides. Chemical Geology, 174, 379–387.
Brun L. A., Maillet, J., Hinsinger, P., & Pepin, M. (2000). Evaluation of copper availability to plants in copper-contaminated vineyard soils. Environmental Pollution, 111, 293–302.
Buschmann, J., & Sigg, L. (2004). Antimony (III) binding to humic substances: Influence of pH and type of humic acids. Environmental Science & Technology, 38, 4535–4541.
Byrd, J. T. (1990). Comparative geochemistries of arsenic and antimony in river and estuaries. The Science of the Total Environment, 97/98, 301–314.
Chang, A. C., Pan, G. X., Page, A. L., & Asano, T. (2002). Developing human health-related chemical guidelines for reclaimed water and sewage sludge applications in agriculture (94 pp). Division of Environmental Health, World Health Organization.
Chinese Society of Soil Sciences. (2000). Analytical methods of soil agricultural chemistry. Beijing: China Agricultural Science and Technology Press.
Coughtrey, P. J., Jackson, D., & Thorne, M. C. (1983). Radionuclide distribution and transport in terrestrial and aquatic ecosystems (Vol. 3). Rotterdam: A.A. Balkema.
Crecelius, E. A., Johnson, C. J., & Hofer, G. C. (1974). Contamination of soils near a copper smelter by arsenic, antimony and lead. Water, Air and Soil Pollution, 3, 337–342.
Crommentuijn, T., Sijm, D., de Bruijn, J., van den Hoop, M., van Leeuwen, K., & van de Plassche, E. (2000). Maximum permissible and negligible concentrations for metals and metalloids in the Netherlands, taking into account background concentrations. Journal of Environmental Management, 60, 121–143.
De Gregori, I., Fuentes, E., Olivares, D., & Pinochet, H. (2004). Extractable copper, arsenic and antimony by EDTA solution from agricultural Chilean soils and its transfer to alfalfa plants (Medicago sativa L.). Journal of Environmental Monitoring, 6, 38–47.
De Gregori, I., Fuentes, E., Rojas, M., Pinochet, H., & Potin-Gautier, M. (2003). Monitoring of copper, arsenic and antimony levels in agricultural soils impacted and non-impacted by mining activities, from three regions in Chile. Journal of Environmental Monitoring, 5, 287–295.
Dietl, C. (1997). Association of antimony with traffic-occurrence in airborne dust, deposition and accumulation in standardized grass cultures. The Science of the Total Environment, 205, 235–244.
DIN 19730 (1997). Bodenbeschaffenheit: Extraktion von Spurenelementen mit Ammoniumnitratlosung, Beuth, Berlin, Germany.
Dodd, M., Pergantis, S. A., Cullen, W. I., Li, H., Eigendor, G. K., & Reimer, K. J. (1996). Antimony speciation in freshwater plant extracts by using hydride generation-gas chromatography-mass spectrometry. Analyst, 121, 223–228.
Eikmann, T., & Kloke A. (1993). Nutzungs- und schutzgutbezogene Orientierungswerte fur (Schad-) stoffe im Boden. In D. Rosenkranz, G. Bachmann, G. Einsele, & H.-M. Harress (Eds.), Bodenschutz. Ergänzbares Handbuch der Maßnahmen und Empfehlungen für Schutz, Pflege und Sanierung von Böden, Landschaft und Grundwasser-1. Band. 14 Lfg. X/93. Berlin, Germany: Erich Schmidt.
Fan, D. L., Zhang, T., & Ye, J. (2004). The Xikuangshan Sb deposit hosted by the Upper Devonian black shale series, Hunan, China. Ore Geology Reviews, 24, 121–133.
Filella, M., Belzile, N., & Chen, Y. W. (2002a). Antimony in the environment: a review focused on natural waters I. Occurrence. Earth-Science Reviews, 57 (1–2), 125–176.
Filella, M., Belzile, N., & Chen, Y. W. (2002b). Antimony in the environment: a review focused on natural waters II. Relevant solution chemistry. Earth-Science Reviews, 59(1–4), 265–285.
Filella, M., Belzile, N., Chen, Y. W., & Deng, T. L. (2003a). Contrasting geochemistry of antimony in lake sediments. Journal De Physique IV, 107, 471–474.
Filella, M., Belzile, N., Chen, Y. W., Elleouet, C., May, P. M., Mavrocordatos, D., Nirel, P., Porquet, A., Quentel, F., & Silver, S. (2003b). Antimony in aquatic systems. Journal De Physique IV, 107, 475–478.
Flynn, H. C., Meharg, A. A., Bowyer, P. K., & Paton, G. I. (2003). Antimony bioavailability in mine soils. Environmental Pollution, 124, 93–100.
Fowler, B. A., Goering, P. L. (1991). Antimony. In E. Merian, (Eds.), Metals and their compounds in the environment. New York, Basel, Cambridge: VCH, Weinheim.
Gebel, T. (1997). Arsenic and antimony: comparative approach on mechanistic toxicology. Chemico-Biological Interactions, 107, 131–144.
Hammel, W., Debus, R., & Steubing (2000). Mobility of antimony in soil and its availability to plants. Chemosphere, 41, 1791–1798.
He, M. C., & Yang, J. R. (1999). Effects of different forms of antimony on the rice during the period of germination and growth and antimony concentration in rice tissue. The Science of the Total Environment, 243/244, 189–196.
He, M. C., & Wan, H. Y. (2004). Distribution, speciation, toxicity and bioavailability of antimony in the environment. Prog Chem, 16(1), 131–135.
He M. C., Xie N. Y., Yu W. D., & Yang R. B. (1994). Study on the effects of antimony in soils on the rice growth and residues (in Chinese). Agro-Environmental Protection (China), 13, 18–22.
Iffland, R. (1988). In H. G. Seiler, H. Sigel, & A. Sigel (Eds.), Handbook on toxicity of inorganic compounds (chap. 7, pp. 67–76). New York: Marcel Dekker.
Jenkins, R. O., Craig, P. J., Goessler, W., Miller, D., Ostah, N., & Irgolic, K. J. (1998). Biomethlation of inorganic antimony compounds by an aerobic fungus: Scopulariopsis brevicaulis. Environmental Science and Technology, 32, 882–885.
Ji, H. B., He, M. C., & Zhao, C. Y. (2003). Research advances of the analytical methods in antimony speciation. Chinese Journal of Analytical Chemistry, 31(11), 1393–1398.
Kabata-Pendias, A., & Pendias, H. (1985). Trace elements in soils and plants. Boca Raton, FL: CRC Press.
Kabata-Pendias, A. (1993). Behavioural properties of trace metals in soils. Applied Geochemistry, 2, 3–9.
Kane, J. S., Arbogast, B., & Leventhal, J. (1990). Characteristics of Devonian Ohio shale SDO-1 as a USGS geochemical reference sample. Geostandards Newsletter, XIV, 169–196.
Koch, I., Wang, L. X., Feldmann, J., Andrewes, P., Reimer, K. J., & Cullen, W. R. (2000). Antimony species in environmental samples. International Journal of Environmental and Analytical Chemistry, 77, 111–131.
Lintschinger, J., Michalke, B., Schulte-Hostede, S., & Schramel, P. (1998). Studies on speciation of antimony in soil contaminated by industrial activity. International Journal of Environmental and Analytical Chemistry, 72, 11–25.
Maiz, I., Arambarri, R., Garcia, R., & Millan (2000). Evaluation of heavy metal availability in contaminated soils by two sequential extraction procedures using factor analysis. Environmental Pollution, 110, 3–9.
Picard, C, & Bosco, M. (2003). Soil antimony pollution and plant growth stage affect the biodiversity of auxin-producing bacteria isolated from the rhizosphere of Achillea ageratum L. FEMS Microbiology Ecology, 46, 73–80.
Qi, W., & Cao, J. (1991). Background concentration of antimony in Chinese soils (In Chinese). Soil Bulletin, 22, 209–210.
Qian, J., Wang, Z., Shan, X., Tu, Q., Wen, B., & Chen, B. (1996). Evaluation of plant availability of soil trace metals by chemical fractionation and multiple regression analysis. Environmental Pollution, 91, 309–315.
Shi, M., Fu, B., Jin, X., & Zhou, X. (1993). The antimony metallogeny in central part of Hunan province (in Chinese with English abstract) (151 pp). Changsha: Hunan Science and Technology Press.
Shotyk, W, Chen, B, & Krachler, M. (2005a). Lithogenic, oceanic and anthropogenic sources of atmospheric Sb to a maritime blanket bog, Myrarnar, Faroe Islands. Journal of Environmental Monitoring, 7, 1148–1154.
Shotyk, W., Krachler, M., & Chen, B. (2005b). Antimony: global environmental contaminant. Journal of Environmental Monitoring, 7, 1135–1136.
Singh, S. P., Tack, F. M., & Verloo, M. G. (1998). Heavy metal fractionation and extractability in dredged sediment derived surface soils. Water, Air and Soil Pollution, 313–328.
Smith, K. S., & Huyck, H. L. O. (1999). An overview of the abundance, relative mobility, bioavailability, and human toxicity of metals. In G. S. Plumlee & M. J. Logsdon (Eds.), The environmental geochemistry of mineral deposits, Part A: Society of Economic Geologists, Reviews in Economic Geology (v. 6A, pp. 29–70).
Taylor, S. R. (1964). Abundance of chemical elements in the continental crust: a new table. Geochimica et Cosmochimica Acta, 28, 1273–1285.
Taylor, S. R., & McLennan, S. M. (1985). The continental crust: Its composition and evolution (312 pp). Oxford: Blackwell Scientific Publications.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51, 844–851.
Tighe, M., Ashley, P., Lockwood, & Wilson, P. S. (2005). Soil, water, and pasture enrichment of antimony and arsenic within a coastal floodplain system. Science of the Total Environment, 347, 175–186.
Wagner, S. E., Peryea, F. J., & Filby, R. A. (2003). Antimony impurity in lead arsenate insecticide enhances the antimony content of old orchard soils. Journal of Environmental Quality, 32, 736–738.
Wilson, N. J., Craw, D., & Hunter, K. (2004). Antimony distribution and environmental mobility at an historic antimony smelter site, New Zealand. Environmental Pollution, 129, 257–266.
Yang G. (1992). Environmental pollution in antimony mine area and the strategy of environmental protection. Paper presented at the 8th annual meeting, Hunan Society of Environmental Sciences and Engineering, Hunan, China.
Yudovich, Ya. E., & Ketris, M. P. (1994). Elements – Impurity in Black Shales (304 pp) (In Russian). Yekaterinburg, Ural: Ural Science Publication.
Acknowledgments
The author is grateful to Dr. Dave Craw (University of Otago) and a second anonymous editor and to the reviewers for their constructive comments. The author was a visiting scholar (2005–2006) in the Department of Geography and Environmental Engineering, Johns Hopkins University and acknowledges Prof. William P. Ball, Johns Hopkins University, for his assistance during the visit and in writing the manuscript. The project was supported by Natural Science Foundation of China (No. 29977002) and National Basic Research Program of China (No. 2004CB418502).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, M. Distribution and phytoavailability of antimony at an antimony mining and smelting area, Hunan, China. Environ Geochem Health 29, 209–219 (2007). https://doi.org/10.1007/s10653-006-9066-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-006-9066-9