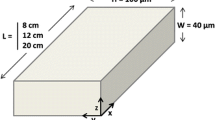
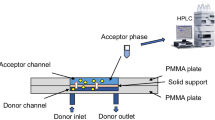
Abstract
Two phases (liquid–liquid extraction) stratified flows in a rectangular geometry are investigated in a double-Y shape glass chip. The influence of physical properties, in particular the viscosity of the two liquids, on the velocity profiles is determined numerically. Viscosity affects the shapes of the velocity profiles of each phase. The extraction conditions (acid concentrations, contact times) in batch and in the microfluidic device were considered for a neutral extractant, N,N′-dimethyl N,N′-dibutyl tetradecylmalonamide (DMDBTDMA) and a liquid anion exchanger (Aliquat® 336), for the extraction of Eu(III) from HNO3 and U(VI) from HCl, respectively. The effects of viscosity and operating conditions on extraction performances were investigated, and the apparent rate constants were evaluated for the extraction of uranium by Aliquat® 336 k app = (0.55 ± 0.03) s−1 and for the extraction of europium by DMDBTDMA: k app = (2.9 ± 0.2) × 10−3 s−1.
















Similar content being viewed by others
Abbreviations
- B o :
-
Bond number
- C a :
-
Capillary number
- \(\mathcal{D}_{\text{M}}\) :
-
Distribution ratio of M in batch
- \({\mathcal{P}}_{\text{M, microsystem}}\) :
-
Distribution ratio of M in microsystem
- D :
-
Diffusion coefficient
- D H :
-
Hydraulic diameter (m)
- E f :
-
Enrichment factor
- g :
-
Gravitational acceleration (m s−2)
- H:
-
Width of the microchannel (m)
- h :
-
Position of the interface (m)
- k app :
-
Initial rate constant (s−1)
- L :
-
Length of the microchannel (m)
- L e :
-
Entrance length (m)
- Q :
-
Flow rate (m2 s−1 in 1D)
- R :
-
Curvature radius of the interface (m)
- Re :
-
Reynolds number
- t :
-
Contact time (s)
- v :
-
Superficial velocity (m s−1)
- \(\left\langle v \right\rangle\) :
-
Average superficial velocity (m s−1)
- W :
-
Depth of the microchannel (m)
- We :
-
Weber number
- V :
-
Volume (m3)
- ρ :
-
Density (kg m−3)
- μ :
-
Dynamic viscosity (Pa s)
- γ a/o :
-
Interfacial tension (N m−1)
- ∇P :
-
Pressure gradient (Pa m−1)
- θ max :
-
Curvature angle of the interface (rad)
- %E :
-
Extraction efficiency (%)
- []:
-
Concentration (mol L−1)
- overbar:
-
In organic phase
- DMDBTDMA:
-
N,N′-dimethyl N,N′-dibutyl tetradecylmalonamide
- R4NCl:
-
Aliquat® 336
- eq:
-
At equilibrium
- a:
-
In aqueous phase
- o:
-
In organic phase
- i:
-
Initial
- exp:
-
Experimental
- th:
-
Theoretical
References
Alcázar A, Perez A, De Lucas A, Carmona M, Rodríguez J (2013) Sulfonation of microcapsules containing selective extractant agents. J Chem Sci Technol 2(2):25–38
Ashcroft SJ, Booker DR, Turner JCR (1990) Density measurement by oscillating tube. Effects of viscosity, temperature, calibration and signal processing. J Chem Soc Faraday Trans 86:145–149
Ban Y, Kikutani Y, Tokeshi M, Morita Y (2011) Extraction of Am(III) at the interface of organic-aqueous two-layer flow in a microchannel. J Nucl Sci Technol 48(10):1313–1318
Barbano PG, Rigali L (1978) Spectrophotometric determination of uranium in sea water after extraction with aliquat-336. Anal Chim Acta 96(1):199–201
Berthon L, Martinet L, Testard F, Madic C, Zemb T (2007) Solvent penetration and sterical stabilization of reverse aggregates based on the DIAMEX process extracting molecules: consequences for the third phase formation. Solvent Ext Ion Exc 25:545–576
Berthon L, Testard F, Martinet L, Zemb T, Madic C (2010) Influence of the extracted solute on the aggregation of malonamide extractant in organic phases: consequences for phase stability. C R Chim 13:1326–1334
Burns JR, Ramshaw C (2001) The intensification of rapid reactions in multiphase systems using slug flow in capillaries. Lab Chip 1:10–15
Ciceri D, Mason L, Harvie D, Perera J, Stevens G (2013) Modelling of interfacial mass transfer in microfluidic solvent extraction: part II. Heterogeneous transport with chemical reaction. Microfluid Nanofluid 14:213–224
DalDon M (1997) Etude des cinétiques d’extraction des nitrates de lanthanides (III) et d’actinides (III) par le diamide dimethyldibutyltetradecylmalonamide. University Paris XI Orsay
Dessimoz AL, Cavin L, Renken A, Kiwi-Minske L (2008) Liquid–liquid two-phase flow patterns and mass transfer characteristics in rectangular glass microreactors. Chem Eng Sci 63:4035–4044
Foroughi H, Kawaji M (2011) Viscous oil–water flows in a microchannel initially saturated with oil: flow patterns and pressure drop characteristics. Int J Multiph Flow 37:1147–1155
Fries DM, Voitl T, Von Rohr PR (2008) Liquid extraction of vanillin in rectangular microreactors. Chem Eng Technol 31(8):1182–1187
Gannaz B, Antonio MR, Chiarizia R, Hill C, Cote G (2006) Structural study of trivalent lanthanide and actinide complexes formed upon solvent extraction. J Chem Soc Dalton Trans 38:4553–4562
Gannaz B, Chiarizia R, Antonio MR, Hill C, Cote G (2007) Extraction of lanthanides(III) and Am(III) by mixtures of malonamide and dialkylphosphoric acid. Solvent Extr Ion Exch 25(3):313–337
Goyal S, Desai V, Lewis RW, Ranganathan DR, Li H, Zeng D, Reichert DE, Kenis P (2014) Thiolene and SIFEL-based microfluidic platforms for liquid–liquid extraction. Sensor Actuat B Chem 190:634–644
Gshneidner KA, Eyring JL, Lander GH, Chopin GC (1994) Lanthanides/actinides: chemistry. In: Nash K (ed) Handbook on the physics and chemistry of rare earth. North Holland, Amsterdam
Hellé G, Mariet C, Cote G (2012) Microfluidic tools for the liquid–liquid extraction of radionuclides in analytical procedures. Procedia Chem 7:679–684
Hibara A, Iwayama S, Matsuoka S, Ueno M, Kikutani Y, Tokeshi M, Kitamori T (2005) Surface modification method of microchannels for gas-liquid two phase flow in microchips. Anal Chem 77(3):943–947
Hisamoto H, Shimizu Y, Uchiyama K, Tokeshi M, Kikutani Y, Hibara A, Kitamori T (2003) Chemico-functional membrane for integrated chemical processes on a microchip. Anal Chem 75:350–354
Horwitz EP (1998) Extraction chromatography of actinides and selected fission products: Principles and achievement of selectivity. International workshop on the application of extraction chromatography in radionuclides measurement. IRMM, Geel
Horwitz EP, McAlister DR, Dietz ML (2006) Extraction chromatography versus solvent extraction: how similar are they. Sep Sci Technol 41:2163–2182
Hotokezaka H, Tokeshi M, Harada M, Kitamori T, Ikeda Y (2005) Development of the innovative nuclide separation system for high-level radioactive waste using microchannel chip-extraction behavior of metal ions from aqueous phase to organic phase in microchannel. Prog Nucl Energy 47(1–4):439–447
Jha MK, Kumar V, Singh RJ (2002) Solvent chloride extraction of zinc from chloride solutions. Solvent Ext Ion Exc 20(3):389–405
Kamholz AE, Yager P (2001) Theoretical analysis of molecular diffusion in pressure-driven laminar flow in microfluidic channels. Biophys J 80:155–160
Kashid MN, Agar DW, Turek S (2007) CFD modelling of mass transfer with and without chemical reaction in the liquid–liquid slug flow microreactor. Chem Eng Sci 62:5102–5109
Kikutani Y, Ueno M, Hisamotoc H, Tokeshi M, Kitamori T (2005) Continuous-flow chemical processing in three-dimensional microchannel network for on-chip integration of multiple reactions in a combinatorial mode. QSAR Comb Sci 24:742–757
Kubota F, Uchida JI, Goto M (2003) Extraction and separation of rare earth metals by a microreactor. Solvent Extr Res Dev Jpn 10:93–102
Lallemand A (1999) Écoulement des fluides : dynamique des fluides reels. Tech Ing BE 8(157):1–22
Logtenberg H, Lopez-Martinez MJ, Feringa BL, Browne WR, Verpoorte E (2011) Multiple flow profiles for two-phase flow in single microfluidic channels through site-selective channel coating. Lab Chip 11:2030–2034
Malengier B, Pushpavanam S, D’haeyer S (2011) Optimizing performance of liquid–liquid extraction in stratified flow in micro-channels. J Micromech Microeng 21(11):115030
Malengier B, Tamalapakula JL, Pushpavanam S (2012) Comparison of laminar and plug flow-fields on extraction performance in micro-channels. Chem Eng Sci 83:2–11
Manz A, Eijkel JC (2001) Miniaturization and chip technology. What can we expect? Pure Appl Chem 73(10):1555–1561
Maruyama T, Uchida JI, Ohkawa T et al (2003) Enzymatic degradation of p-chlorophenol in a two-phase flow microchannel system. Lab Chip 3:308–312
Maruyama T, Matsushita H, Uchida JI, Kubota F, Kamiya N, Goto M (2004) Liquid membrane operations in a microfluidic device for selective separation of metal ions. Anal Chem 76:4495–4500
Mason LR, Ciceri D, Harvie DJE, Perera JM, Stevens GW (2013) Modelling of interfacial mass transfer in microfluidic solvent extraction: part I. Heterogenous transport. Microfluid Nanofluid 14:197–212
Morita K, Hagiwara T, Hirayama N, Imura H (2010) Extraction of Cu(II) with dioctyldithiocarbamate and a kinetic study of the extraction using a two-phase microflow system. Solvent Extr Res Dev Jpn 17:209–214
Nishihama S, Nishimura G, Hirai T, Komasawa I (2004) Separation and recovery of Cr(VI) from simulated plating waste using microcapsules containing quaternary ammonium salt extractant and phosphoric acid extractant. Ind Eng Chem Res 43:751–757
Nishihama S, Tajiri Y, Yoshizuka K (2006) Separation of lanthanides using micro solvent extraction system. Ars Separatoria Acta 4:18–26
Rice N, Irving H, Leonard M (1993) Nomenclature for liquid–liquid distribution (Solvent extraction). Pure Appl Chem 65(11):2373–2396
Sagar S, Deshpande SS, Joshi JB, Kumar VR, Kulkarni BD (2008) Identification and characterization of flow structures in chemical process equipment using multiresolution techniques. Chem Eng Sci 63(21):5330–5346
Salim A, Fourar M, Pironon J, Sausse J (2008) Oil–water two-phase flow in microchannels: flow patterns and pressure drop measurements. Can J Chem Eng 86(6):978–988
Sato T (1983) Liquid-liquid extraction of rare-earth elements from aqueous acid solutions by acid organophosphorus compounds. Hydrometallurgy 22:121–140
Tabeling P (2003). Introduction à la microfluidique. Belin
Tokeshi M, Kitamori T (2005) Continuous flow chemical processing on a microchip using operations and a multiphase flow network. Prog Nucl Energy 47(1–4):434–438
Tokeshi M, Minagawa T, Kitamori T (2000) Integration of a microextraction system on a glass chip: ion-pair solvent extraction of Fe(II) with 4,7-diphenyl-1,10-phenanthrolinedisulfonic acid and tri-n-octylmethylammonium chloride. Anal Chem 72:1711–1714
Tsaoulidis D, Dore V, Angeli P, Plechkova NV, Seddon KR (2013a) Extraction of dioxouranium(VI) in small channels using ionic liquids. Chem Eng Res Des 91:681–687
Tsaoulidis D, Dore V, Angeli P, Plechkova NV, Seddon KR (2013b) Flow patterns and pressure drop of ionic liquid–water two-phase flows in microchannels. Int J Multiph Flow 54:1–10
Weigl M, Geist A, Gompper K, Kim J (2001) Kinetics of lanthanide/actinide co-extraction with N, N′-dimethyl-N, N′ dibutyltetradecylmalonic diamide (DMDBTDMA). Solvent Ext Ion Exc 19(2):215–229
Whitesides GM (2006) The origins and the future of microfluidics. Nature 442(7101):368–373
Zhao T (2009) Microfuel cells. Elsevier, San Diego
Zhao Y, Chen G et al (2006) Liquid-liquid two-phase flow patterns in a rectangular microchannel. Am Inst Chem Eng J 52(12):4052–4060
Zhao Y, Chen G et al (2007) Liquid–liquid two-phase mass transfer in the T-junction microchannels. Am Inst Chem Eng J 53(12):3042–3053
Znidarsic-Plazl Z, Plazl I (2007) Steroid extraction in a microchannel system—mathematical modelling and experiments. Lab Chip 7:883–889
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hellé, G., Mariet, C. & Cote, G. Liquid–liquid microflow patterns and mass transfer of radionuclides in the systems Eu(III)/HNO3/DMDBTDMA and U(VI)/HCl/Aliquat® 336. Microfluid Nanofluid 17, 1113–1128 (2014). https://doi.org/10.1007/s10404-014-1403-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-014-1403-1