Abstract
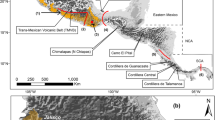
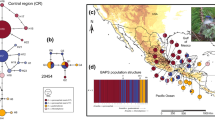
High diversity, complex topography and geological factors make the Neotropics an interesting region for the study of avian diversification. Phylogeography, in conjunction with ecological niche modeling, offers an interesting complementary approach to identify the tempo and mode of diversification in different species. In this work, we analyzed the genetic structure of Habia fuscicauda based on multilocus analyses using mtDNA (ND2 and ND4) and nuDNA (ACOI-I9 and ODC) sequences. Additionally, we transferred the optimal conditions for current distribution to the Mid-Holocene, Last Glacial Maximum and the Last Inter-Glacial in order to evaluate the shift in species distribution and compare with the genetic data. Our results indicate that H. fuscicauda comprises two clades: one with genetic correspondence to south-east Mexico to the Nicaragua Depression and the other from the south of the Nicaragua Depression to Central Panama. Within these clades, we identified genetic differentiation between populations across the Isthmus of Tehuantepec, Costa Rica—associated with the Talamanca Cordillera—and Central Panama. The two principal clades showed contrasting demographic histories, with the northern clade showing demographic changes and the southern clade demographic stasis. The ecological niche models identified areas as refugia for the northern clade fin Central America and for the southern clade in the Talamanca Cordillera and Central Panama, supporting the hypothesis that these clades were isolated from each other during the climate shifts of the Pleistocene.
Zusammenfassung
Die Phylogeographie des Rotkehlkardinals (Habia fuscicauda, Cardenalidae) zeigt Populationen-Isolierung, genetische Divergenz und demographische Veränderungen während der Klimaveränderungen im mittelamerikanischen Regenwald des Quartärs. Eine hohe Diversität, komplexe Topographie sowie geologische Faktoren machen die Neotropen zu einer interessanten Region für die Untersuchung der Artenbildung bei Vögeln. Die Phylogeographie bietet in Verbindung mit der ökologischen Nischenmodellierung einen interessanten ergänzenden Ansatz, um die Geschwindigkeit und die Art und Weise der Ausbildung verschiedener Arten zu untersuchen. In dieser Arbeit haben wir die genetische Struktur des Rotkehlkardinals (Habia fuscicauda) anhand von Multi-Locus-Sequenzanalysen unter Verwendung von mtDNA- (ND2 und ND4) und nuDNA- (ACOI-I9 und ODC) Sequenzen analysiert. Zusätzlich dazu übertrugen wir die optimalen Bedingungen für die derzeitige Verbreitung auf das Mittelholozän, das Letzteiszeitliche Maximum und die letzte Zwischeneiszeit, um die Verschiebung der Artenverteilung zu bewerten und mit den genetischen Daten zu vergleichen. Unsere Ergebnisse deuten darauf hin, dass H. fuscicauda zwei Kladen umfasst: eine mit genetischer Entsprechung im Südosten Mexikos bis zum Nicaragua-Graben und die zweite vom Süden des Nicaragua-Grabens bis nach Zentralpanama. Innerhalb dieser Kladen konnten wir genetische Unterschiede zwischen Populationen am Isthmus von Tehuantepec, in Costa Rica,—in Verbindung mit der Talamanca-Gebirgskette—und in Zentralpanama feststellen. Die beiden Hauptkladen zeigten gegensätzliche demographische Verläufe, wobei die nördliche Klade demographische Veränderungen und die südliche Klade einen demographischen Stillstand aufwies. Die ökologischen Nischenmodelle identifizierten bestimmte Gebiete als Rückzugsorte für die nördliche Klade in Mittelamerika und für die südliche Klade in der Talamanca-Gebirgskette und Zentralpanama, was die Hypothese unterstützt, dass diese Kladen während der Klimaverschiebungen im Pleistozän isoliert voneinander waren.






Similar content being viewed by others
Availability of data and materials
The sequences generated during the current study are available in GenBank (accession numbers: MW884324–MW884530).
Code availability
Not applicable.
References
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F (Eds) Proceedings of the 2nd international symposium on information theory, Budapest, Akademiai Kiado, pp 267–281.
Arbeláez-Cortés E (2012) Comparative phylogeography: concepts, methods and general patterns in neotropical birds. Acta Biol Colomb 17:19–38
Arbeláez-Cortés E, Nyári ÁS, Navarro-Sigüenza AG (2010) The differential effect of lowlands on the phylogeographic pattern of a Mesoamerican montane species (Lepidocolaptes affinis, Aves: Furnariidae). Mol Phylogenet Evol 57:658–668
Arévalo E, Davis SK, Sites JW (1994) Mitochondrial DNA sequence divergence and phylogenetic relationships among eight chromosome races of the Sceloporus grammicus complex (Phrynosomatidae) in central Mexico. Syst Biol 43:387–418
Avise JC, Arnold J, Ball RM, Bermingham E, Lamb T, Neigel JE, Reeb CA, Saunders NC (1987) Intraspecific phylogeography: the mitochondrial DNA bridge between population genetics and systematics. Annu Rev Ecol Evol Syst 18:489–522
Bagley JC, Johnson JB (2014) Phylogeography and biogeography of the lower central american neotropics: diversification between two continents and between two seas. Biol Rev 89:767–790
Ball RM, Avise JC (1992) Mitochondrial DNA phylogeographic differentiation among avian populations and the evolutionary significance of subspecies. Auk 109:626–636
Bandelt H, Forster P, Rohl A (1999) Median joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48
Barber BR, Klicka J (2010) Two pulses of diversification across the Isthmus of Tehuantepec in a montane Mexican bird fauna. Proc R Soc B 277:2675–2681
Barker FK, Burns KJ, Klicka J, Lanyon SM, Lovette IJ (2015) New insights into New World biogeography: an integrated view from the phylogeny of black- birds, cardinals, sparrows, tanagers, warblers, and allies. Auk 132:333–348
Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, Gavryushkina A, Heled J, Jones G, Kühnert D, De Maio N, Matschiner M, Mendes FK, Müller NF, Ogilvie HA, du Plessis L, Popinga A, Rambaut A, Rasmussen D, Siveroni I, Suchard MA, Wu C-H, Xie D, Zhang C, Stadler T, Drummond JA (2019) BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Comput Biol 15:e1006650
Burney CW (2009) Comparative phylogeography of Neotropical birds. LSU Doctoral Dissertations. 2682.
Burns KJ, Hackett SJ, Klein NK (2003) Phylogenetic relationships of Neotropical honeycreepers and the evolution of feeding morphology. J Avian Biol 34:360–370
Carnaval AC, Moritz C (2008) Historical climate modelling predicts patterns of current biodiversity in the Brazilian Atlantic forest. J Biogeogr 35:1187–1201
Carnaval AC, Hickerson MJ, Haddad CFB, Rodrigues MT, Moritz C (2009) Stability predicts genetic diversity in the Brazilian Atlantic forest hotspot. Science 323:785–789
Castillo-Chora VdeJ, Sánchez-González LA, Mastretta-Yanes A, Prieto-Torres DA, Navarro-Sigüenza AG (2021) Insights into the importance of areas of climatic stability in the evolution and maintenance of avian diversity in the Mesoamerican dry forests. Biol J Linn Soc Lond 132:741–758
Cheviron Z, Hackett SJ, Capparella AP (2005) Complex evolutionary history of a neotropical lowland forest bird (Lepidothrix Coronata) and its implications for historical hypotheses of the origin of neotropical avian diversity. Mol Phylogenet Evol 36:338–357
D’Horta FM, Cuervo AM, Ribas CC, Brumfield RT, Miyaki CY (2013) Phylogeny and comparative phylogeography of Sclerurus (Aves: Furnariidae) reveal constant and cryptic diversification in an old radiation of rain forest understorey specialists. J Biogeogr 40:37–49
Dinerstein E, Olson D, Joshi A, Vynne C, Burgess ND, Wikramanayake E, Hahn N, Palminteri S, Hedao P, Noss R, Hansen M, Locke H, Ellis EC, Jones B, Barber CV, Hayes R, Kormos C, Martin V, Crist E, Sechrest W, Price L, Baillie JEM, Weeden D, Suckling K, Davis C, Sizer N, Moore R, Thau D, Birch T, Potapov P, Turubanova S, Tyukavina A, de Souza N, Pintea L, Brito JC, Llewellyn OA, Miller AG, Patzelt A, Ghazanfar SA, Timberlake J, Klöser H, Shennan-Farpón Y, Kindt R, Barnekow Lillesø J-P, van Breugel P, Graudal L, Voge M, Al-Shammari KF, Saleem M (2017) An ecoregion-based approach to protecting half the terrestrial realm. Bioscience 67:534–545
dos Anjos L, Debus SJS, Madge SC, Marzluff JM (2009) Family Corvidae (Crows). In: del Hoyo J, Elliott A, Christie DA (eds) Handbook of the birds of the world: bush-shrikes to old world sparrows, vol 14. Lynx Edicions, Barcelona, pp 678–588
Drummond AJ, Rambaut A, Shapiro B, Pybus OG (2005) Bayesian coalescent inference of past population dynamics from molecualr sequences. Mol Biol Evol 22:1185–1192
Drummond AJ, Suchard MA, Xie D, Rambaut A (2013) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973
Dwight J, Griscom L (1924) Descriptions of new birds from Costa Rica. Am Mus Novit 142:1–5
Edler D, Klein J, Antonelli A, Silvestro D (2020) raxmlGUI 2.0: a graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol Evol 00:1–5
Elith J, Graham CH, Anderson RP, Dudık M, Ferrier S, Guisan A, Hijmans RJ, Huettmann F, Leathwick JR, Lehmann A, Li J, Lohmann LG, Loiselle BA, Manion G, Moritz C, Nakamura M, Nakazawa Y, McC J, Overton M, Peterson AT, Phillips SJ, Richardson K, Scachetti-Pereira R, Schapire RE, Soberón J, Williams S, Wisz MS, Zimmermann NE (2006) Novel methods improve prediction of species’ distributions from occurence data. Ecography 29:129–151
Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ (2011) A statistical explanation of MaxEnt for ecologists. Divers Distrib 17:43–57
ESRI (2010) ArcGIS desktop. Environmental Systems Research Institute, Redlands
Excoffier L, Schneider S (1999) Why hunter-gatherer populations do not show signs of Pleistocene demographic expansions. PNAS 96:10597–10602
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform 1:47–50
Excoffier L, Foll M, Petit RJ (2009) Genetic consequences of range expansions. Annu Rev Ecol Evol Syst 40:481–501
Flores-Villela O, Martínez-Salazar EA (2009) Historical explanation of the origin of the herpetofauna of Mexico. Rev Mex Biodivers 80:817–833
Fu YX, Li WH (1993) Statistical tests of neutrality of mutations. Genetics 133:693–709
Gernhard T (2008) The conditioned reconstructed process. J Theor Biol 253:769–778
González C, Ornelas JF, Gutiérrez-Rodríguez C (2011) Selection and geographic isolation influence hummingbird speciation: genetic, acoustic and morphological divergence in the wedge-tailed sabrewing (Campylopterus curvipennis). BMC Evol Biol 11:38
Grant WS, Bowen BW (1998) Shallow population histories in deep evolutionary lineages of marine fishes: insights from sardines and anchovies and lessons for conservation. J Hered 89:415–426
Haffer J (1969) Speciation in Amazonian forest birds. Science 165:131–137
Harpending RC (1994) Signature of ancient population growth in a low-resolution mitochondrial DNA mismatch distribution. Hum Biol 66:591–600
Harvey MG, Brumfield RT (2015) Genomic variation in a widespread neotropical bird (Xenops Minutus) reveals divergence, population expansion, and gene flow. Mol Phylogenet Evol 83:305–316
He L, Zhang A, Weese D, Zhu C, Jiang C, Qiao Z (2010) Late pleistocene population expansion of Scylla Paramamosain along the Coast of China: a population dynamic response to the last interglacial sea level highstand. J Exp Mar Biol Ecol 385:20–28
Hewitt GM (1996) Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc 58:247–276
Hewitt GM (2000) The genetic legacy of the quaternary ice ages. Nature 405:907–913
Hewitt GM (2004) Genetic consequences of climatic oscillations in the Quaternary. Philos Trans R Soc Lond B Biol Sci 359:183–195
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hilty S (2011) Red-throated Ant-tanager (Habia fuscicauda). In: del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E (eds) Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona
Ibrahim KM, Nichols RA, Hewitt GM (1996) Spatial patterns of genetic variation generated by different forms of dispersal during range expansion. Heredity 77:282–291
Jackson JCB, Budd AF, Coates AG (1996) Evolution and environment in tropical America. University of Chicago Press, Chicago
Jezkova T, Riddle BR, Card DC, Schield DR, Eckstut ME, Castoe TA (2015) Genetic consequences of postglacial range expansion in two codistributed rodents (genus Dipodomys) depend on ecology and genetic locus. Mol Ecol 24:83–97
Kimball RT, Braun EL, Barker FK, Bowie RCK, Braunm MJ, Chojnowski JL, Hackett SJ, Han KL, Harshman J, Heimer-Torres V, Holznagel W, Huddlestone CJ, Marks BD, Miglia KJ, Moore WS, Reddy S, Sheldon FH, Smith JV, Witt CC, Yuri T (2009) A well-tested set of primers to amplify regions spread across the avian genome. Mol Phylogenet Evol 50:654–660
Klicka J, Burns K, Spellman GM (2007) Defining a monophyletic Cardinalini: a molecular perspective. Mol Phylogenet Evol 45:1014–1032
Lavinia PD, Escalante P, García NC, Barreira AS, Trujillo-Arias N, Tubaro PL, Naoki K, Miyaki CY, Santos FR, Lijtmaer DA (2015) Continental-scale analysis reveals Deep diversification within the polytypic Red-crowned Ant Tanager (Habia rubica, Cardinalidae). Mol Phylogenet Evol 89:182–193
Leigh JW, Bryant D (2015) PopART: full-feature software for haplotype network construction. Methods Ecol Evol 6(9):1110–1116
Li WH, Sadler LA (1991) Low nucleotide diversity in man. Genetics 129:513–523
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Licona-Vera Y, Ornelas JF (2014) Genetic, ecological and morphological divergence between populations of the endangered Mexican Sheartail hummingbird (Doricha eliza). PLoS ONE 9:e101870
Liu J-X, Gao T-X, Yokogawa K, Zhang Y-P (2006) Differential population structuring and demographic history of two closely related fish species, Japanese Sea Bass (Lateolabrax japonicus) and spotted Sea Bass (Lateolabrax maculatus) in Northwestern Pacific. Mol Phylogenet Evol 39:799–811
Lobo JM, Jiménez-Valverde A, Real R (2008) AUC: a misleading measure of the performance of predictive distribution models. Glob Ecol Biogeogr 17:145–151
Lovette IJ (2005) Glacial cycles and the tempo of avian speciation. Trends Ecol Evol 20:57–59
Maldonado-Sánchez D, Gutiérrez-Rodríguez C, Ornelas JF (2016) Genetic divergence in the common bush-tanager Chlorospingus ophthalmicus (Aves: Emberizidae) throughout Mexican cloud forests: the role of geography, ecology and Pleistocene climatic fluctuations. Mol Phylogenet Evol 99:76–88
Marshall C, Liebherr J (2000) Cladistic biogeography of the Mexican Transition Zone. J Biogeogr 27:203–216
Mendoza AM, Bolívar-García W, Vázquez-Domínguez E, Ibáñez R, Parra Olea G (2019) The role of Central American barriers in shaping the evolutionary history of the northernmost glassfrog, Hyalinobatrachium fleischmanni (Anura: Centrolenidae). PeerJ 7:e6115
Miguez-Gutiérrez A, Castillo J, Márquez J, Goyenechea I (2013) Biogeografía de la Zona de Transición Mexicana con base en un análisis de árboles reconciliados. Rev Mex Biodivers 84:215–224
Miller MA, Pfeifeer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the gateway computing envirnonments workshop (GCE), New Orleans, LA: 1–8.
Moore WS (1995) Inferring phylogenies from mtDNA variation: mitochondrial-gene trees versus nuclear-gene trees. Evolution 49:718–726
Morando M, Avila LJ, Turner C, Sites JW (2008) Phylogeography between valleys and mountains: the history of populations of Liolaemus koslowskyi (Squamata, Liolaemini). Zool Scr 37:603–618
Mulcahy D, Benson G, Morrill H, Mendelson JR (2006) Historical biogeography of Lowland Species of Toads (Bufo) across the Trans-Mexican Neovolcanic Belt and the Isthmus of Tehuantepec. J Biogeogr 33:1889–1904
Muscarella R, Galante PJ, Soley-Guardia M, Boria RA, Kass JM, Uriarte M, Anderson RP (2014) ENMeval: an R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol Evol 5:1198–1205
Oberholser HC (1922) Phoenicothraupis Cabanis becomes Habia Blyth. P Biol Soc Wash 35:79–80
Ornelas JF, Sosa V, Soltis DE, Daza JM, González C, Soltis PS, Gutiérrez-Rodríguez C, Espinosa de los Monteros A, Castoe TA, Bell C, Ruiz-Sanchez E (2013) Comparative phylogeographic analyses illustrate the complex evolutionary history of threatened cloud forests of Northern Mesoamerica. PLoS ONE 8:2
Ottenburghs J, Kraus RHS, van Hooft P, van Wieren SE, Ydenberg RC, Prins HHT (2017) Avian introgression in the genomic era. Avian Res 8:30
Owens HL, Campbell L, Dornak L, Saupe E, Barve N, Soberón J, Ingenloff K, Lira-Noriega A, Hensz CM, Myers CE, Peterson AT (2013) Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol Model 263:10–18
Pennington RT, Prado DE, Pendry CA (2000) Neotropical seasonally dry forests and Quaternary vegetation changes. J Biogeogr 27:261–273
Pennington RT, Lavin M, Prado DE, Pendry CA, Pell SK, Butterworth CA (2004) Historical climate change and speciation: neotropical seasonally dry forest plants show patterns of both Tertiary and Quaternary diversification. Philos Trans R Soc Lond B Biol Sci 359:515–538
Peterson AT, Papeş M, Soberón J (2008) Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol Model 213:63–72
Phillips SJ, Dudik M (2008) Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31:161–175
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Prado DE, Gibbs PE (1993) Patterns of species distributions in the dry seasonal forests of South America. Ann Mo Bot Gard 80:902–927
Primmer CR, Borge T, Lindell J, Saetre GP (2002) Single-nucleotide polymorphism characterization in species with limited available sequence information: high nucleotide diversity revealed in the avian genome. Mol Ecol 11:603–612
Puebla-Olivares F, Bonaccorso E, Espinosa de los Monteros A, Omland KE, Llorente-Bousquets JE, Peterson AT, Navarro-Sigüenza AG (2007) Speciation in the Emerald Toucanet (Aulacorhynchus prasinus) complex. Auk 125:39–50
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/.
Rambaut A, Drummond AJ (2013) Tracer v1.6.0. http://beast.bio.ed.ac.uk/Tracer.
Ramírez-Barrera SM, Hernández-Baños BE, Jaramillo-Correa JP, Klicka J (2018) Deep divergence of Red-crowned Ant Tanager (Habia rubica: Cardinalidae), a multilocus phylogenetic analysis with emphasis in Mesoamerica. PeerJ 6:e5496
Rocha-Méndez A, Sánchez-González LA, Arbeláez-Cortés E, Navarro-Sigüenza AG (2018) Phylogeography indicates incomplete genetic divergence among phenotypically differentiated montane forest populations of Atlapetes albinucha (Aves, Passerellidae). Zookeys 809:125–148
Rogers AR, Harpending H (1992) Population growth makes waves in the distribution of pairwise genetic differences. Mol Biol Evol 9:552–569
Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian inference and model choice across a large model space. Syst Biol 61:539–542
Rull V (2008) Speciation timing and neotropical biodiversity: the tertiary–quaternary debate in the light of molecular phylogenetic evidence. Mol Ecol 17:2722–2729
Silva T, Guzmán A, Urantówka AD, Mackiewicz P (2017) A new parrot taxon from the Yucatán Peninsula, Mexico—its position within genus Amazona based on morphology and molecular phylogeny. PeerJ 5:e3475
Slatkin M (1993) Isolation by distance in equilibrium and non-equilibrium populations. Evolution 47:264–279
Slatkin M, Hudson RR (1991) Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. Genetics 129:555–562
Smith BT, Klicka J (2010) The profound influence of the Late Pliocene Panamanian uplift on the exchange, diversification, and distribution of New World birds. Ecography 33:333–342
Smith BT, Ribas CC, Whitney BM, Hernández-Baños BE, Klicka J (2013) Identifying biases at different spatial and temporal scales of diversification: a case study in the Neotropical parrotlet genus Forpus. Mol Ecol 22:483–494
Soberón J, Peterson AT (2005) Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers Inf 2:1–10
Sobral-Souza T, Lima-Ribeiro MS, Solferini VN (2015) Biogeography of Neotropical Rainforests: past connections between Amazon and Atlantic Forest detected by ecological niche modeling. Evol Ecol 29:643–655
Sorenson MD, Ast CJ, Dimcheff DE, Yuri T, Mindell DP (1999) Primers for PCR-based approach to mitocondrial genome sequencing in birds and other vertebrates. Mol Phylogenet Evol 12:105–114
Soubiès F, Suguio K, Martin L (1991) The Quaternary lacustrine deposits of the Serra dos Carajás (State of Pará, Brazil): ages and other preliminary results. Bol IG-USP 8:223–243
Stohlgren TJ, Otsuki Y, Villa CA, Lee M, Belnap J (2001) Patterns of plant invasions: a case example in native species hotspots and rare habitats. Biol Invasions 3:37–50
Suzuki R, Shimodaira H (2006) Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 22:1540–1542
Tajima F (1989a) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Tajima F (1989b) The effect of change in population size on DNA polymorphism. Genetics 123:597–601
Templeton AR, Maskas SD, Cruzan MB (2000) Gene trees: a powerful tool for exploring the evolutionary biology of species and speciation. Plant Species Biol 15:211–222
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Villar-Anleu L (1994) Informe de País Guatemala: Perfil General. In Vega A (Ed). Corredores Conservacionistas en la Región Centroamericana: Memorias de una Conferencia Regional auspiciada por el Proyecto Paseo Pantera. Florida: Tropical Research and Development, Inc.
Xie WG, Lewis PO, Fan Y, Kuo L, Chen MH (2011) Improving marginal likelihood estimation for bayesian phylogenetic model selection. Syst Biol 60:150–160
Yule GU (1925) A Mathematical theory of evolution based on the conclusions of Dr. J C Willis. Philos Trans R Soc B 213:21–87
Zink RM, Klicka J, Barber BR (2004) The tempo of avian diversification during the Quaternary. Philos Trans R Soc B 359:215–220
Acknowledgements
VJC-C thanks the Posgrado en Ciencias Biológicas from the Universidad Nacional Autónoma de México (UNAM) and CONACYT for the Doctoral scholarship received to perform this research, as well as the support of the scholarship “Ayudante de Investigador SNI III” (EXP. AYTE. 16804).
Funding
The research was supported by PAPIIT/DGAPA, Universidad Nacional Autónoma de México (UNAM) through a grant to Blanca E. Hernández-Baños (IN220620).
Author information
Authors and Affiliations
Contributions
BEHB and VJC-C designed the study. BEHB secured financial support. VJC-C carried out the laboratory work. BEHB, LEZB, and VJC-C analyzed the data. BEHB, VJC-C, LEZB and CP contributed to the writing and improvement of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Consent to participate
The authors declare their consent to participate.
Consent for publication
The authors declare their consent for publication of the manuscript.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki.
Additional information
Communicated by J. T. Lifjeld.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10336_2021_1904_MOESM1_ESM.docx
Supplementary file 1. (DOCX 69974 KB). Table SM1. GenBank accession numbers for the analyzed data and downloaded sequences used as external groups. Table SM2. Prior distribution for calibration of the main nodes used to estimate divergence times for Habia fuscicauda in Beast. Table SM3. Clean occurrence dataset for the Ecological Niche Model analysis. Table SM4. Analyses of molecular variance (AMOVA) between geographical groups NCA and LCA. Figure SM1. Model selection of bioclimatic variables and tuning of the best parameter combination for the Ecological Niche Model. a) Clustering bios in a dendrogram with 1,000 bootstrap replicates with approximately unbiased (AU) probability values (p-values). b) correlation matrix heatmap: Positive correlations are displayed in blue and negative correlations in red color. Color intensity and the size of the circle are proportional to the correlation coefficients. c) Tuning of the best parameter combination of regularization multiplier and feature types through AICc model selection. Bios: bio1 = Annual Mean Temperature; bio2 = Mean Diurnal Range; bio3 = Isothermality; bio4 = Temperature Seasonality; bio5 = Max Temperature of Warmest Month; bio6 = Min Temperature of Coldest Month; bio7 = Temperature Annual Range; bio8 = Mean Temperature of Wettest Quarter; Mean Temperature of Driest Quarter; bio10 = Mean Temperature of Warmest Quarter; bio11 = Mean Temperature of Coldest Quarter; bio12 = Annual Precipitation; bio13 = Precipitation of Wettest Month; bio14 = Precipitation of Driest Month; bio15 = Precipitation Seasonality; bio16 = Precipitation of Wettest Quarter; bio17 = Precipitation of Driest Quarter; bio18 = Precipitation of Warmest Quarter; bio19 = Precipitation of Coldest Quarter. Figure SM2. mtDNA and nDNA phylogenetic trees. Color tips indicate populations according to geographic delimitations. Polygons or symbols indicate the subspecies corresponded to each individual. Support values indicate posterior probability for Bayesian approach and bootstrap support for Maximum Likelihood. Figure SM3. Haplotype networks for the individual genes a) ND4 (mtDNA), b) ACOI-I9 (nDNA), and c) ODC (nDNA). Colors used for geographic delimitation of populations are the same as in the main text.
Rights and permissions
About this article
Cite this article
Castillo-Chora, V.d., Zamudio-Beltrán, L.E., Pozo, C. et al. Phylogeography of Habia fuscicauda (Cardinalidae) indicates population isolation, genetic divergence and demographic changes during the Quaternary climate shifts in the Mesoamerican rainforest. J Ornithol 162, 961–976 (2021). https://doi.org/10.1007/s10336-021-01904-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-021-01904-x